Abstract
Chronic myelomonocytic leukemia (CMML) is a clonal hematopoietic malignancy that may deserve specific management. Defined by a persistent peripheral blood monocytosis ≥1 × 109/L and monocytes accounting for ≥10% of the white blood cells, this aging-associated disease combines cell proliferation as a consequence of myeloid progenitor hypersensitivity to granulocyte-macrophage colony-stimulating factor with myeloid cell dysplasia and ineffective hematopoiesis. The only curative option for CMML remains allogeneic stem cell transplantation. When transplantation is excluded, CMML is stratified into myelodysplastic (white blood cell count <13 × 109/L) and proliferative (white blood cell count ≥13 × 109/L) CMML. In the absence of poor prognostic factors, the management of myelodysplastic CMML is largely inspired from myelodysplastic syndromes, relying on erythropoiesis-stimulating agents to cope with anemia, and careful monitoring and supportive care, whereas the management of proliferative CMML usually relies on cytoreductive agents such as hydroxyurea, although ongoing studies will help delineate the role of hypomethylating agents in this patient population. In the presence of excessive blasts and other poor prognostic factors, hypomethylating agents are the preferred option, even though their impact on leukemic transformation and survival has not been proved. The therapeutic choice is illustrated by 4 clinical situations among the most commonly seen. Although current therapeutic options can improve patient’s quality of life, they barely modify disease evolution. Improved understanding of CMML pathophysiology will hopefully lead to the exploration of novel targets that potentially would be curative.
Introduction
In patients with a peripheral blood (PB) monocytosis, defined by a monocyte count >1 × 109/L, a clonal hematopoietic disorder must be considered as soon as reactive causes have been excluded. Chronic myelomonocytic leukemia (CMML), a clonal hematopoietic malignancy characterized by persistent monocytosis, combines myeloid cell proliferation with myeloid cell dysplasia and ineffective hematopoiesis, and has thus been classified by the World Health Organization (WHO) as a myelodysplastic syndrome (MDS)/myeloproliferative neoplasm (MPN).1 This disease, which demonstrates a propensity to transform into acute myeloid leukemia (AML), has long been considered an MDS. Most of the efforts to delineate the specificities of CMML and dedicated therapeutic strategies started recently.
The disease incidence rate ranges between 3.5 and 4.1/1 000 000 per year in the United States and in Europe.2-5 Median age at diagnosis is 72 years, CMML being rare in young adults. Whole genome sequencing of leukemic cell DNA identified 2 age-related molecular signatures,6 supporting the idea that aging is the main cause of the disease. CMML demonstrates a male predominance (2.3 males for 1 female), which is more apparent among older patients. The clinical, biological, and pathological expression of CMML is heterogeneous; latent forms may be neglected and the disease incidence underestimated.
How we diagnose CMML
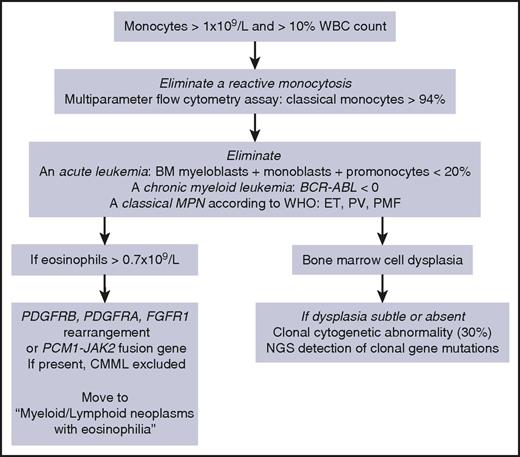
According to the WHO,1 CMML diagnosis requires a persistent monocytosis with monocytes accounting for ≥10% of the white blood cell (WBC) differential count (Table 1, “WHO-defined criteria”). Other disorders must be excluded: the percentage of blast cells in the bone marrow (BM) and the PB, including myeloblasts, monoblasts, and promonocytes, must be <20%; BCR-ABL1 rearrangement and WHO criteria for essential thrombocytosis, polycythemia vera, and primary myelofibrosis must be absent; in rare persistent monocytosis with eosinophilia, a rearrangement involving PDGFRA (4q12), PDGFRB (5q31-33), FGFR1 (8p11-12), or JAK2 (9p24, PCM1-JAK2 fusion) gene must be excluded cytogenetically. Dysplasia in 1 or more myeloid lineages is commonly observed on BM aspirate, but it can be absent or subtle.1 In this latter situation, the diagnosis may be made if an acquired clonal cytogenetic or molecular genetic abnormality is detected in hematopoietic cells (Figure 1).1 Cytogenetic abnormalities are detected in ∼30% of patients, the most frequent being trisomy 8, loss of chromosome Y, monosomy 7, deletion 7q, trisomy 21, and del(20q).6
Our diagnostic workup for CMML diagnosis. ET, essential thrombocytosis; NGS, next-generation sequencing; PV, polycythemia vera; PMF, primary myelofibrosis.
Our diagnostic workup for CMML diagnosis. ET, essential thrombocytosis; NGS, next-generation sequencing; PV, polycythemia vera; PMF, primary myelofibrosis.
Molecular aberrations affect mostly epigenetic, splicing, and signaling genes.7 Typically, the disease genomic fingerprint combines mutations in TET2, SRSF2, and ASXL1 and genes of the RAS signaling pathway (NRAS, KRAS, CBL).8 The association of SRSF2 and TET2 mutations may be highly specific for CMML.9 In clinical practice, sequencing of a limited number of genes detects a clonal abnormality in >90% of cases (Table 2).8-11
In the absence of myeloid cell dysplasia, CMML is diagnosed when monocytosis persists for at least 3 months and other causes of monocytosis are excluded.1 A flow cytometry assay that analyzes monocyte subsets allows exclusion of a benign reactive monocytosis without waiting for 3 months (Table 1, “Additional criteria”). The expression of CD14 and CD16 distinguishes classical (CD14+/CD16−), intermediate (CD14+/CD16+), and nonclassical (CD14−/CD16+) monocytes. In healthy conditions, up to 94% of monocytes are classical monocytes. CMML is characterized by an increase in this fraction ≥94%, whereas benign reactive monocytosis is due to the accumulation of the 2 minor subsets (Figure 2).12-14 Of interest, this abnormal monocyte subset repartition can be observed in a fraction of MDS patients, with half of them developing a monocytosis in the following year.15
Monocyte subset repartition in patients with a monocytosis. Multiparameter flow cytometry analysis of monocyte (MO) subsets13 in a healthy donor and in the 4 reported cases, showing an increased fraction of classical monocytes (>94%), except in case report 2 in which an associated inflammatory disease alters the characteristic repartition.
Monocyte subset repartition in patients with a monocytosis. Multiparameter flow cytometry analysis of monocyte (MO) subsets13 in a healthy donor and in the 4 reported cases, showing an increased fraction of classical monocytes (>94%), except in case report 2 in which an associated inflammatory disease alters the characteristic repartition.
With the improvement of diagnostic tools, CMML is increasingly recognized following the incidental finding of an elevated PB monocyte count. Clinical expression of CMML otherwise includes varied combinations of constitutional symptoms, clinical effects of cytopenias or spleen size increase, extramedullary myelomonocytic infiltrates, and associated autoimmune and inflammatory diseases.16,17 The PB leukocytosis may include monocyte or neutrophil predominance, anemia and thrombocytopenia are common and sometimes immune related, and leukocytosis requesting urgent therapy and severe neutropenia are rare. In a hypercellular BM, dysplasia is inconstant, blast cell percentage is heterogeneous, and reticulin fibrosis, nodules of plasmacytoid dendritic cells, ring sideroblasts, and mast cell infiltrates can be observed.18-21 This heterogeneous presentation was recently opposed to a lower genetic heterogeneity.22
How we stratify CMML risk
The median survival of CMML patients ranges from <1 year in population-based studies of older patients2 to almost 3 years in other subgroups.7,8,11 Leukemic transformation rate varies between 15% and 30%.7,8,11 It is therefore useful to detect at diagnosis the patients at high risk of rapid death or transformation into hard-to-treat AML.
The WHO recognizes 2 prognostic parameters.1 A WBC count ≥13 × 109 g/L separates MPN-CMML, in which the RAS/MAPK signaling pathway is frequently activated, from MDS-CMML, whose outcome is better.1,8 The other is blast cell percentage with 3 groups: CMML-0 (<2% blasts in PB and <5% in BM), CMML-1 (2% to 4% in PB and/or 5% to 9% in BM), and CMML-2 (5% to 19% blasts in PB and/or 10% to 19% in BM and/or Auer rods are present). The distinction between promonocytes, which must be counted as blast cells, dysplastic monocytes, and dysplastic granulocytes requires expertise.23,24
A number of prognostic scoring systems have been established.8,11,25-32 The International Prognostic Scoring System developed to stratify MDS excludes proliferative CMML.33 A combination of clinical, cytogenetic, and molecular information may improve the accuracy of CMML prognostication.7,8,34,35 Three cytogenetic categories were identified as independent prognostic variables: low risk (normal karyotype or loss of Y), high risk (trisomy 8 or abnormal chromosome 7 or complex karyotype), and intermediate risk (the others).6 These groups were introduced in a CMML-specific prognostic scoring system.31 Interrogation of several models in a common database showed that all the scoring systems that do not include genetic information have comparable performance.36 We have shown that, in addition to older age, elevated WBC, and low platelet count, ASXL1 frameshift or nonsense mutations (but not missense mutations) were an independent predictor of poor survival.8 Recently, cytogenetic abnormalities and mutations in ASXL1, RUNX1, NRAS, and SETBP1 were independently associated with overall survival, providing a genetic score that, combined with red blood cell transfusion dependency, WBC, and marrow blasts, defines 4 risk groups.11 Detailed comparison with previously established prognostication tools using a common, large, and robust data set, is now mandatory to define an international, consensus scoring system. Meanwhile, any of the described stratification tools may be used for clinical decision-making in routine practice.
CMML patient management
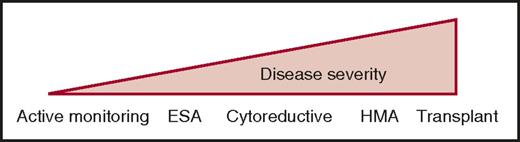
Except for allogeneic stem cell transplantation (ASCT), there is no disease-modifying treatment in CMML. Current therapies aim to improve symptom burden using a personalized strategy guided by cytopenia-induced or proliferation-associated symptoms. Commonly used drugs include erythropoiesis-stimulating agents (ESAs) in anemic patients, cytoreductive drugs in proliferative diseases, and hypomethylating agents (HMAs) in the most severe CMML, especially when cytopenias predominate (Figure 3). The therapeutic choice is illustrated by 4 common clinical situations.
Current therapeutic strategies in CMML. Given the clinical and biological heterogeneity of the disease, current treatment goes from a watch-and-wait attitude with active monitoring of symptoms and complications resulting from HMAs or allogeneic stem cell transplantation (transplant), depending on age and comorbidities. Cytoreductive, usually hydroxyurea.
Current therapeutic strategies in CMML. Given the clinical and biological heterogeneity of the disease, current treatment goes from a watch-and-wait attitude with active monitoring of symptoms and complications resulting from HMAs or allogeneic stem cell transplantation (transplant), depending on age and comorbidities. Cytoreductive, usually hydroxyurea.
How we manage a stable, low-grade CMML?
Case report 1 (part 1)
A 60-year-old man with a medical record of asthma and peptic ulcer was fortuitously diagnosed with isolated monocytosis. His physician observed a stable monocyte count ∼2 × 109/L. After 2 years, the patient was referred to a hematology department. His physical examination was normal. His blood cell count showed a normal WBC count (6.7 × 109/L) and confirmed the monocytosis (1.6 × 109/L) with normal hemoglobin level (135 g/L) and platelet count (184 × 109/L). His BM aspirate was normocellular, showing 1% blasts, 8% monocytes, and minimal signs of dysplasia, including hypolobulated megakaryocytes and some dysgranulopoiesis. His karyotype was normal.
Stable isolated monocytosis
According to the WHO criteria, this patient has a typical CMML-0.1 His age younger than 60 years; the absence of elevated leukocytosis, anemia, or thrombocytopenia; the lack of blast excess in the BM; and circulating immature myeloid cells are good prognostic factors.7,8,36 Flow cytometry analysis of PB monocyte subsets13,14 and molecular analysis of a panel of genes using new-generation sequencing7,8,10 could now complete the evaluation. Given his young age, ASCT could be considered if the disease evolves. In the absence of any progression and poor prognostic factor, we recommend a wait-and-watch attitude because a small fraction of CMML remains stable for many years.
Case report 1 (part 2)
The patient has been seen twice a year in the outpatient unit for 6 years without any therapeutic intervention. His blood cell count remains stable. Flow cytometry analysis of his PB monocytes showed a typical increase in the classical monocyte fraction (Figure 2), and molecular analyses detected mutations in TET2 and SRSF2. These results enforced CMML diagnosis without modifying the watch-and-wait attitude.
How we manage CMML in an inflammatory setting
Case report 2 (part 1)
An 80-year-old woman was referred for anemia. She had a history of Hashimoto thyroiditis and complained of chronic inflammatory pain in the cervical spine and shoulders. Her blood cell results revealed a normal WBC count (5.1 × 109/L) with a normal neutrophil count (1.87 × 109/L) but an increased monocyte count (1.87 × 109/L), a mild normocytic anemia (hemoglobin 93 g/L, mean cell volume 89 fL), and thrombocytopenia (112 × 109/L). C reactive protein (12 mg/L) and fibrinogen level (4.6 g/L) were increased. Immune tests were negative for antinuclear and anti–double-stranded DNA antibodies and rheumatoid factor. Her serum electrophoresis showed 2 small spikes. Upon physical examination, there was no joint swelling, no spleen or liver enlargement, and no serous effusion or skin lesion. The PB smear showed pseudo-Pelger-Huët neutrophils with hypogranulation, red cell anisocytosis, and poikilocytosis. BM aspirate showed an average cellularity, normal megakaryocytes, lack of monocyte excess (3%), hypogranulated promyelocytes and myelocytes, and a few agranular blast cells (1%). Plasma cells (2%) were morphologically normal. The karyotype of BM cells was normal.
Cytopenias in CMML
At CMML diagnosis, the hemoglobin level is <100 g/L in ∼40% of patients, with 25% having red cell transfusion requirements. Anemia impairs overall survival and is incorporated in diverse scoring systems.8,11 Clinical experience and individual cases suggested that ESAs provided comparable results in MD-CMML and MDS.37,38 A recent retrospective analysis of their efficacy demonstrated that a response was observed in ∼60% of patients and red cell transfusion independence in ∼30%. A better response was detected in anemic patients with low endogenous serum erythropoietin level and transfusion independence.39 Thrombocytopenia is common in CMML patients because of megakaryocyte dysplasia40,41 or autoimmune mechanisms.42-44 The orally bioavailable, small nonpeptide thrombopoietin receptor agonist eltrombopag demonstrates some efficacy. Because safety concerns have been raised,45 this drug should be used only in clinical trials (Table 3).
CMML and lymphoid malignancies
A polyclonal hypergammaglobulinemia is commonly observed in CMML patients,46,47 likely reflecting elevated levels of inflammatory cytokines. The combination of a monoclonal gammopathy of undetermined significance, a multiple myeloma, or a lymphoid malignancy with CMML is rare.47-52 Of note, TET2 mutations can be detected in both myeloid and lymphoid malignancies.53 In TET2-mutated lymphoid malignancies, the detection of a mutated allele in immature myeloid progenitors suggests that a common genetic background could generate both myeloid and lymphoid diseases.54,55
Case report 2 (part 2)
Flow cytometry analysis of PB monocytes showed that classical monocytes represented only 73.2% of total monocytes, resulting from an increased fraction of intermediate monocytes (Figure 2). Sequencing of monocyte DNA identified somatic mutations in TET2, SRSF2, and RUNX1. A diagnosis of CMML-0 was made. One year later, while polymyalgia rheumatica was controlled by prednisone and a sustained erythroid response to ESA had been obtained, her WBC count increased to 16 × 109/L. Without circulating immature myeloid cells, she developed a splenomegaly, and the thrombocytopenia worsened to 32 × 109/L with easy bruising. A control BM aspirate showed no blast excess or new cytogenetic lesion.
Inflammation, autoimmune diseases, and CMML
Retrospective studies suggest that the prevalence of inflammatory diseases (10% to 30%), which can be diagnosed concomitantly or shortly before or after CMML, is increased.56,57 It remains difficult to determine if they are the ground for CMML or if CMML predisposes to these diseases.58-60 The most frequently observed include systemic vasculitis, connective tissue diseases, polychondritis, seronegative arthritis, and immune thrombocytopenia.17,42-45,61 In such an inflammatory setting, the typical CMML phenotype can be transiently masked by an increased fraction of CD14+,CD16+ intermediate monocytes (Figure 1). When the inflammatory disease is controlled (eg, with steroids), the phenotype comes back to a typical accumulation of classical monocytes. If uncertainty exists, a search for somatic mutations in PB cells by sequencing a panel of frequently mutated genes (Table 3) allows practitioners to confidently determine whether a patient with normal karyotype and subtle dysplasia truly has a clonal disease. First-line treatment of inflammatory and immune diseases consists mostly of steroids, with ∼85% response. Steroid dependence and recurrence are observed in ∼50% of cases. Most additional immunosuppressive therapies may increase the risk of severe cytopenia and infections. However, HMAs, which have immunomodulatory functions, may be efficient and decrease steroid amounts and steroid dependence.43,62 It remains unclear if these events impact CMML overall survival.
How we manage a proliferative CMML in a young patient
Case 3 report (part 1)
A 41-year-old male was referred by a primary care facility for a newly diagnosed CMML. This house painter had been a heavy smoker for 20 years. His father had died of lung cancer, and 1 of his 3 sisters had been treated for breast cancer, but he had no family history of hematopoietic malignancy. The diagnosis of CMML was made after a 3-year follow-up for thrombocytopenia. His PB count showed an increased WBC count (29.6 × 109/L), including monocytosis (4.1 × 109/L) with decreased hemoglobin level (108 g/L) and platelet count (52 × 109/L). Neutrophils were increased (12.7 × 109/L), with 14% immature myeloid cells and 9% peripheral blasts. His physical examination was normal except for bruises. His BM aspirate showed 12% blasts, including promonocytes and features of dysgranulopoiesis; his karyotype was normal.
CMML in younger patients
Although CMML patients younger than 65 years have a better survival,8 median survival remains short (months) and treatment outcome is better with ASCT than with HMAs.63 CMML cases in young adults can develop on a background of germ line mutations in RUNX1,41 GATA2,64 ANKRD26,65 or DDX41.66 Preferred interactions between mutational events may explain the frequent acquisition of somatic ASXL1 on a GATA2-mutated background.67 About 10% of CMMLs develop after exposure to chemotherapies or radiation, with a median latency of 6 years,68 These therapy-related CMMLs could be the accelerated expansion of a clonal hemopoiesis of indeterminate potential.69
Case 3 report (part 2)
Molecular testing on BM mononucleated cells collected at diagnosis revealed 2 mutations in RUNX1, 1 being a stop gain (variant allele frequency [VAF], 48%) whose somatic origin was uncertain, the other being subclonal. Additional somatic mutations were in TET2, SRSF2, ASXL1, NRAS, and ZRSR2 (VAF, 27% to 44%). He also had somatic mutations in BCOR and PHF6, with VAFs in the 75% to 85% range, compatible with these genes being located on the X chromosome. In the days following referral, the patient’s WBC count increased to 39 g/L. The 2 healthy sisters were haploidentical to the patient. Databases indicated a high probability of finding a matched unrelated donor. Based on BM and PB blast excess, normal cytogenetics and high matched unrelated donor probability, the patient was treated with an intensive chemotherapy regimen. Eight-week assessment showed a hypocellular marrow with the persistence of a blast excess (7%). The patient received fludarabine/melphalan–conditioned ASCT from a matched unrelated donor. Transplant was complicated with grade 2 acute graft-versus-host disease. CMML relapsed 8 months posttransplantation, with a 10% blast excess in the BM.
ASCT
ASCT remains the unique potentially curative therapy and the preferred therapeutic option in younger patients with high-risk CMML. It is therefore essential to assess the disease risk at diagnosis, using WHO criteria and 1 of the currently established scoring systems. A minority of high-risk patients is eligible, owing to advanced age and comorbidities. The advent of reduced intensity conditioning and alternate donor sources may increase the number of transplanted patients. All of the studies so far have been retrospective. Except a few recent reports,70,71 most have included a small number of patients.72-77 Current recommendations are therefore based on expert opinion rather than evidence.78,79 The median age of transplanted patients ranges from 50 to 56 years. About one-third of these patients are alive a few years later. Deaths are due almost equally to treatment-related mortality or posttransplant disease relapse. Indirect evidence for a graft-versus-CMML effect comes from correlations between graft-versus-host disease and reduction in relapse.71 The main risk factor for transplant-related mortality is the disease status. Although there is no consensus about the correct timing of ASCT in CMML patients, transplantation should preferably be performed early after diagnosis and after establishing the best possible remission status.70 There is no evidence whether the use of cytoreductive therapy before transplant could improve the outcome compared with frontline ASCT. Expert opinion suggests treatment before transplantation, especially when marrow blast cells are >10% and in patients with high-risk CMML according to available scoring systems.78,79 The best treatment of reducing tumor burden before transplantation, either intensive chemotherapy or HMAs, remains a controversial issue.80,81 Many experts currently consider pretreatment with an HMA in the case of CMML-2, even though evidence from prospective clinical trials is lacking. Some transplant candidates are eventually not transplanted owing to pretreatment toxicity or disease progression before transplant.
How we manage myeloproliferative CMML in an older patient
Case report 4 (part 1)
A 75-year-old male with a long history of high blood pressure, arrhythmia, and arteritis was referred for an elevated WBC count (27.6 × 109/L) and thrombocytopenia (50 × 109/L). WBC included a majority of neutrophils (10.5) and monocytes (11.8 × 109/L) including 5% circulating immature myeloid cells. The hemoglobin level was normal (140 g/L). Physical examination detected a splenomegaly (16 cm craniocaudal length by ultrasonography). BM aspiration detected 11% blast cells, including myeloblasts and promonocytes, indicating a CMML-2. Cytogenetic analysis of BM cells showed an isolated monosomy 7. Molecular analyses detected somatic mutations in ASXL1, ETV6, KRAS, NRAS, RIT1, SRSF2, and TET2. The patient was considered ineligible for transplant based on age and comorbidities.
Myeloproliferative CMML
A fraction of CMML patients demonstrate a rise in their peripheral leukocyte count, which can be dramatic without overt transformation into AML. This increase can be transiently majored by an associated infectious or inflammatory disease. In juvenile myelomonocytic leukemia, constitutive activation of the Ras pathway82,83 induces a hypersensitivity of myeloid progenitors to granulocyte-macrophage colony-stimulating factor (GM-CSF) with a specifically evoked STAT5 signature.84 In CMML, most of the disease-associated molecular events also converge within a GM-CSF/pSTAT5 pathway.85,86 Spontaneous formation of colony-forming unit GM at disease diagnosis could be a surrogate parameter of RAS pathway hyperactivation that correlates with a worse outcome.87,88 Therapeutic approaches targeting this signaling pathway are currently being assessed,89 including the use of ruxolitinib that could control inflammatory symptoms (Table 3).90
Extramedullary disease
MPN-CMML patients can experience constitutional symptoms, including fatigue, diffuse bone pain, and night sweats, and develop a splenomegaly (∼30% of cases), which is occasionally troublesome, resulting from extramedullary hematopoiesis.91 Extramedullary disease, which occasionally infiltrates the skin,92,93 lymph nodes,94,95 gingiva,96 kidneys,97 pericardium,98 and other sites,99,100 could regress upon treatment with hydroxyurea,96 methylprednisolone,101 or HMA.102-104 Nevertheless, splenomegaly is a poor prognostic factor in CMML treated with HMAs.105
Cytoreductive therapy
Oral cytoreductive agents have long been the main treatment of proliferative CMML. They remain required frontline in patients with initial leukocytosis >50 × 109/L, severe constitutional symptoms, splenomegaly, or extramedullary hematopoiesis.99,105 Even when HMAs are initiated, an initial cytoreduction may be required.106 A randomized trial demonstrated that the outcome was better in patients treated with hydroxyurea compared with oral etoposide.105 Low-risk CMML patients with MPN-like features can be effectively managed with hydroxyurea. There is no formal demonstration that control of myeloproliferative features improves outcome, and molecular studies have shown continuing clonal evolution under hydroxyurea,86 but this phenomenon has also been reported with HMA.6 There is no recommended target WBC or monocyte count, but we usually try to reduce WBC count below 15 × 109/L. Cytarabine is used occasionally as a debulking agent. Induction chemotherapy with anthracyclines and cytarabine or newer regimens including clofarabine have been used to bridge to ASCT.107
HMAs
Clinical trials that established the efficacy of azacitidine (AZA) and decitabine (DAC) in MDS included a limited number of CMML patients.29,80,108 Small dedicated clinical trials showed overall response rates according to International Working Group (IWG) criteria109 ranging between 30% and 60%, with a complete response in fewer than 15% of patients. Median overall survival ranged between 12 and 37 months.106,110-112 AZA is licensed for nonproliferative CMML-2 in many countries; DAC is licensed in the United States only. These drugs are globally well tolerated, with myelosuppression being the major toxicity. Responses are generally not sustained but, in the absence of alternative, treatment is maintained until progression. Survival after loss of response is dismal.113 Somatic mutations are not predictive of treatment efficacy.106,114 Gene expression pattern, DNA methylation pattern, and platelet doubling after the first cycle could be more informative.106,114-116
Case 4 report (part 2)
The patient was treated with DAC on a conventional schedule in the ongoing Decitabine with or without Hydroxyurea versus Hydroxyurea in CMML trial (EudraCT: 2014-000200-10). After 6 cycles, he was in complete response according to IWG criteria. After 12 cycles, he developed grade 2 thrombocytopenia at day 28 of each cycle, lowering to 20 g/L at day 28 of cycle 16. The BM aspirate was of average cellularity with few megakaryocytes and persistent dysgranulopoiesis, without excess of blasts or monocytes. Cycle 17 was delayed by 4 weeks, and further cycles spaced every 6 weeks. The patient remains stable after 21 cycles of DAC at 2 years from diagnosis.
The limits of HMA in CMML
Although HMAs demonstrate cytotoxic effects, their epigenetic activity plays a central role in restoring a balanced hematopoiesis. AZA and DAC induce dramatic changes in DNA methylation and gene expression in leukemic cells of responding patients.116 However, they do not reduce the mutated allele burden, nor do they permit the reexpansion of wild-type hematopoietic cells. In addition, the response to AZA or DAC does not prevent the accumulation of genetic damage in the leukemic clone.6
Response criteria
IWG response criteria for MDS are not completely suited for adult patients with MDS/MPN106 (eg, they do not capture the treatment effects on the proliferative component). Also, scales that have not been specifically developed for CMML barely reflect the drug effects on constitutional symptoms.90 An international consortium recently proposed specific end points to better measure benefit to the patient.21 The proposed criteria may capture meaningful improvements not seen with IWG criteria, but the more stringent definition of complete response leads to divergent response rates compared with IWG criteria.117
Currently explored therapeutic strategies in CMML patients
Because there is an unmet need for therapeutic options that modify CMML course and the disease specificities are increasingly identified, new therapeutic approaches are explored (Table 3). One objective may be to restore a wild-type hematopoiesis. Many drugs have been tested in cohorts of MDS to which CMML was added, which may preclude identification of CMML-specific effects.118 Short homogeneous series of CMML patients have been treated with recombinant α-2b interferon, which reduced monocytosis without improving cytopenias,119 or with all-trans retinoic acid, which improved cytopenias without reducing proliferation.120 Homogeneous cohorts of CMML deserving therapy remain difficult to recruit.
Guadecitabine is a dinucleotide of DAC and deoxyguanosine resists deamination by cytidine deaminase. The half-life of DAC after subcutaneous injection of guadecitabine is longer than that of DAC delivered intravenously.121 A phase 2 study is ongoing in patients with high-risk MDS (Table 3). Because HMAs could increase the expression of surface immune checkpoint proteins at the surface of MDS and CMML CD34+ cells,122 a clinical trial explores the potential of immune checkpoint inhibitors in combination with AZA in a cohort of MDS and CMML patients. IDH1 and IDH2 gene mutations are detected in only a small fraction of CMML patients,123 but their presence can be suspected by detecting an elevated level of 2-hydroxyglutarate in the patient serum124 ; the currently developed IDH inhibitors could be an option in these situations.125
Molecular aberrations detected in CMML converge within the GM-CSF signaling pathway.85 A human GM-CSF fused to a truncated diphtheria toxin selectively kills leukemic progenitors of CMML patients but has never been developed clinically.126 Lenzilumab, a recombinant monoclonal antibody that neutralizes GM-CSF binding to its cognate receptor and could control cell proliferation, is currently being tested.85 GM-CSF hypersensitivity of CMML myeloid progenitors correlates with mutations in genes of the Ras signaling pathway. The farnesyltransferase inhibitor tifiparnib127 is currently being tested in a phase 2 trial. An alternative could be to inhibit Ras effectors that include the MEK/extracellular signal-regulated kinase and the phosphoinositide-3′-OH kinase/Akt cascades.128-130 Chemical inhibitors of JAK2, a sentinel kinase in this pathway,85 could inhibit the growth of CMML myeloid progenitors,131 and ruxolitinib was shown to be safe to decrease the size of the spleen and to induce responses that were not restricted to patients with somatic mutations in signaling genes while improving constitutional symptoms.90 Pacritinib, a JAK2/FLT3 inhibitor, also deserves to be tested in proliferative CMML.132,133
Because activation of the Hedgehog signaling pathway could contribute to the development of leukemic stem cells, CMML patients can be included in a phase 1 study of a smoothened antagonist.134 Finally, high expression of interleukin-3 receptor (CD123) in leukemic stem cells led exploration of the efficacy of SL-401, a diphtheria toxin-interleukin-3 fusion protein,135 in patients with hematological malignancies including CMML. Another characteristic feature of CMML is the frequent mutations of a splicing gene.6,8 The gain-of-function mutation in SRSF2 results in transcriptome-wide missplicing,136 and cells with a mutated allele are more sensitive to splicing inhibitory molecules than wild-type cells,137 providing a therapeutic window that is explored in clinics.
Regarding cytopenias, transforming growth factor-β modulators such as sotatercept are explored to treat anemia,138 whereas trials testing eltrombopag in thrombocytopenic patients are under way. Proliferative CMML could develop leukocytosis with circulating blast cells upon therapy, an effect whose long-term consequences has to be evaluated.62
Finally, molecules that target the mature cells of the clone have been identified preclinically. An adenosine-containing cyclic dinucleotide induces the selective apoptosis of monocytes, in vitro and in vivo,139 whereas trabectedin, a DNA minor groove binder that has been tested in solid tumor patients, could selectively deplete myelomonocytic cells by inhibiting their growth and triggering their apoptosis.140
Conclusions and perspectives
Stepwise linear regression models suggest that somatic mutations account for only 15% to 24% of variability of clinical phenotypes.11 These mutations occur in the hematopoietic stem and progenitor cell compartment and accumulate linearly in these cells with clonal dominance in this compartment.141 Therapies may aim at eradicating the founder mutant clones in this cell compartment and restore a wild-type hematopoiesis. Azanucleosides restore a balanced hematopoiesis but fail to decrease the mutation allele burden in leukemic cells, suggesting that epigenetic alterations account for CMML phenotypes.6 Mature cells of the leukemic clone, including monocytes, dysplastic granulocytes, and others,22 may contribute to disease installation or progression through the cytokines they produce.142-144 Finally, the role played by the microenvironment145 and the macroenvironment, including gut microbiota146 in this disease, remains unknown. Current therapeutic approaches improve the patient quality of life, but we have still to identify a disease-modifying therapeutic approach.
Acknowledgments
The authors thank the patients with chronic myelomonocytic leukemia who agree to participate in their investigations and the physicians who collect information and send biological samples for analyses. Most of these physicians are members of the Groupe Francophone des Myelodysplasies. The authors are also grateful to Dorothée Selimoglu-Buet for providing illustrations of flow cytometry analyses and to Jeffie Lafosse for collecting clinical information.
This work is supported by the Ligue Nationale Contre le Cancer (équipe labellisée).
Authorship
Contribution: E.S. and R.I. conceived and wrote the manuscript.
Conflict-of-interest disclosure: E.S. received research grants from CIT Pharma and Servier. R.I. declares no competing financial interests.
Correspondence: Eric Solary, Gustave Roussy Cancer Center, 114 rue Edouard Vaillant, 94805 Villejuif, France; e-mail: eric.solary@gustaveroussy.fr; or Raphael Itzykson, Hôpital Saint-Louis, 1, Ave Claude Vellefaux, 75010 Paris, France; e-mail: raphael.itzykson@aphp.fr.