Abstract
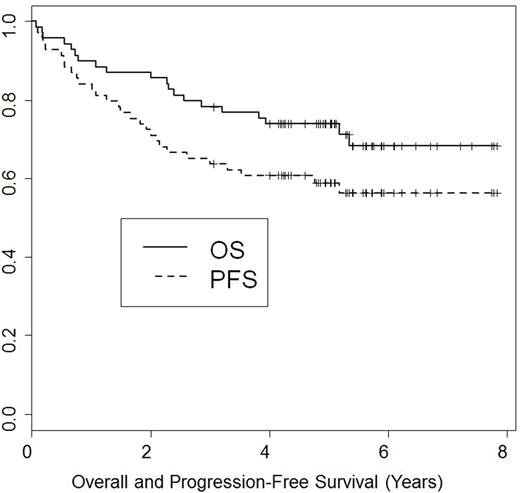
Background: We previously reported favorable 2-year outcomes of NMA transplantation after BFR conditioning in patients with relapsed lymphoid malignancies. In this report, we evaluated the long-term outcomes of this treatment. Methods: Patients were enrolled on two consecutive clinical trials. Patients received bendamustine 130 mg/m2 IV daily on days -5 to - 3 prior to transplantation, together with 30 mg/m2 IV of fludarabine given on the same days. Rituximab was given at a dose of 375 mg/m2 IV on day -13 and 1000 mg/m2 on days -6, +1, and +8. Rituximab was omitted in patients with T-cell diseases. Tacrolimus and mini-methotrexate were used for GvHD prophylaxis. In addition, thymoglobulin 1 mg/kg IV was given on days -2, and -1 in patients receiving an unrelated donor transplant. Results: Sixty- nine consecutive patients were enrolled between April 2009 and February 2013. Histologies included: CLL (n=28) (8 with 17p- and 2 Richter's), follicular (n=13), mantle cell (n=16), diffuse large cell (n=9), peripheral t-cell lymphoma (n=3). Median age was 59 (range, 30-72) years. Median prior treatments was 3 (range, 1-7); 7 (10%) had failed a prior autotransplant. At transplantation, 29 (42%) patients were in CR/CRu, 31 (46%) in PR, and 8 (12%) had refractory disease. Twenty (29%) patients were PET+, and 19 (27.5%) had elevated LDH. Thirty-six (52%) received their transplants from HLA-compatible siblings and 33 (48%) from unrelated donors. Median number of CD34-positive cells infused was 5.58 x 106/kg. Neutrophil counts recovered to > 0.5 x 109/L a median of 0 days after transplantation (range, 0-17 days). Seventeen patients (25%) never experienced an ANC < 0.5 x 109/L. Fifty-eight patients (84%) never experienced a platelet count of < 20 x 109/L. All patients engrafted donor cells. By day 30, median donor myeloid and T-cells were 92% and 97%, respectively. Both increased to 100% by day 90. The cumulative incidence of grade 2-4 GvHD and chronic extensive GvHD were 17% and 31%, respectively. Treatment-related mortality at 1-year was 9%. Nineteen (27.5%) patients died: 9 of progression, 6 of infection and 4 of GvHD complications. With a median follow-up time of 5 years (range, 0.1-8 years), the 5-year OS rate was 74% [82% CLL/Richter's, 85% follicular, 67% diffuse large and t-cell, and 56% in mantle cell (p=0.43)] and the 5-year PFS rate was 60% (Figure). Conclusions: Our mature results confirm the earlier observations that the BFR regimen constitutes a well-tolerated NMA allogeneic conditioning for lymphoid malignancies. It allows engraftment of sibling and unrelated donor cells with minimal myelosuppression. Promising long-term survival results were seen in patients with both indolent and aggressive histologies.
Khouri: Novartis: Research Funding. Jabbour: Bristol-Myers Squibb: Consultancy. Oran: Astex: Research Funding; Celgene: Research Funding; AROG: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.