Abstract
Background
The management of patients with the Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs) polycythemia vera (PV) and myelofibrosis (MF) is evolving. US clinical practice guidelines for MF were published for the first time in late 2016, and those for PV (and essential thrombocythemia) are in development. Clinicians still face substantial challenges in selecting therapy for patients with PV and MF. To assist with patient care and to help healthcare providers (HCPs) make informed decisions in this complex treatment landscape, an online treatment decision support tool was developed. Here we report data comparing expert treatment recommendations from the tool with the intended treatment entered by HCPs using the tool.
Methods
In February 2017, 5 experts in MPN patient care provided specific treatment recommendations for numerous distinct PV and MF case scenarios. These scenarios were defined by key patient and disease characteristics, including the presence of disease symptoms or anemia, previous response to treatment, and eligibility for transplantation and other therapeutic modalities. To use the tool, participating HCPs entered specific patient and disease factors along with their intended treatment plan for their case. When the case entry was completed, the expert treatment recommendations for that specific patient case were shown, followed by a short survey designed to determine if the expert recommendations had changed the HCP's planned course of treatment.
Results
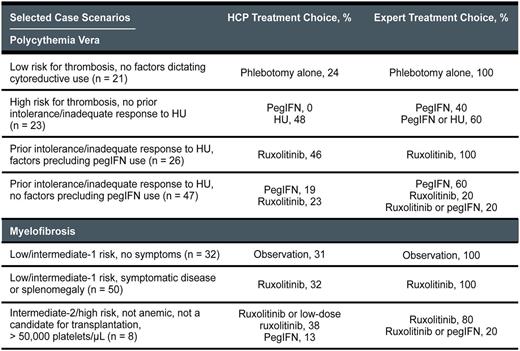
From March 20, 2017, to June 9, 2017, 369 patient cases (n = 172 for PV; n = 197 for MF) were entered into the tool by 270 HCPs (83% MDs). Although there was expert consensus (≥ 3 of 5 experts choosing the same treatment course) for the majority of PV and MF cases (table), variance among the experts did exist in select areas: for example, the treatment of anemic patients with intermediate-2-risk/high-risk MF who had significant disease symptoms or splenomegaly but were not candidates for transplantation. A comparison of expert and tool user treatment choices showed substantial variability for several different patient case scenarios (table). For example, compared with expert recommendations for the PV cases, many tool users would potentially overtreat patients at low risk for thrombosis and underuse peginterferon (pegIFN) and ruxolitinib for high-risk patients. Approximately one half of the PV cases entered into the tool involved patients with prior intolerance/inadequate response to hydroxyurea (HU), and the intended treatment for these patients indicated by the majority of HCPs did not match consensus expert recommendations. Compared with expert recommendations for the MF cases, many tool users would potentially overtreat asymptomatic low-risk/intermediate-1-risk patients and underuse ruxolitinib for higher-risk patients. Among the HCPs who reported that their intended treatment plan differed from the experts, 73% indicated that the recommendations provided by the tool would change their previous management plans.
Conclusions
Analysis of cases and intended treatment plans entered into an online PV/MF treatment decision support tool indicated variance in expert and HCP treatment choices for many case scenarios. Expert recommendations in this online tool changed the intended treatment plan of many HCPs and, as such, suggest that online treatment decision tools developed by respected experts can help optimize the care of patients with PV and MF. A full analysis of all cases entered into the tool-including a detailed report of expert and HCP practice trends for PV and MF management-will be presented.
Deininger: Ariad Pharmaceuticals, Bristol Myers Squibb, CTI BioPharma Corp, Gilead, Incyte, Novartis, Pfizer, Celgene, Blue Print, Galena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Research Funding; ARIAD: Consultancy; Incyte: Consultancy; Celgene: Research Funding; Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Pfizer: Consultancy. Mascarenhas: Promedior: Research Funding; Incyte: Other: Clinical Trial Steering Committee , Research Funding; CTI Biopharma: Research Funding; Janssen: Research Funding; Merck: Research Funding; Novartis: Other: DSMB member , Research Funding. Mesa: Incyte Corporation: Research Funding; Ariad: Consultancy; Promedico: Research Funding; Celgene Corporation: Research Funding; Galena Biopharma, Inc.: Consultancy; Gilead Sciences, Inc.: Research Funding; CTI BioPharma Corp.: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy. Stein: Incyte: Consultancy. Verstovsek: Lilly Oncology: Research Funding; Promedior: Research Funding; Astrazeneca: Research Funding; Pfizer: Research Funding; Incyte: Research Funding; Bristol Myers Squibb: Research Funding; Pfizer: Research Funding; Gilead: Research Funding; Roche: Research Funding; Astrazeneca: Research Funding; NS Pharma: Research Funding; Galena BioPharma: Research Funding; CTI BioPharma Corp: Research Funding; Bristol Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Gilead: Research Funding; NS Pharma: Research Funding; Celgene: Research Funding; Promedior: Research Funding; Roche: Research Funding; Seattle Genetics: Research Funding; CTI BioPharma Corp: Research Funding; Lilly Oncology: Research Funding; Genentech: Research Funding; Celgene: Research Funding; Genentech: Research Funding; Blueprint Medicines Corp: Research Funding; Blueprint Medicines Corp: Research Funding; Galena BioPharma: Research Funding; Incyte: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.