Abstract
Indolent non-Hodgkin's lymphoma (iNHL) and mantle cell lymphoma (MCL) represent a diverse group of treatable but incurable B-cell lymphoid malignancies. Since 2013, bendamustine and rituximab (BR) followed by maintenance rituximab (MR) became standard upfront therapy for patients with treatment naïve iNHL and transplant ineligible MCL at CancerCare Manitoba, a tertiary referral center in the province of Manitoba, Canada. The aim of this retrospective study is to describe efficacy of BR, outside of a clinical trial, with a focus on toxicity.
Manitoba provincial oncology drug program database was used to identify patients. Treatment naïve iNHL and transplant-ineligible MCL patients treated with BR were included. Chronic lymphocytic leukemia, transformed and aggressive B-cell lymphoma patients were excluded. Patient and disease characteristics including response evaluation, dates of relapse, death and hematologic and non-hematologic toxicities were collected. Statistical analysis was completed using univariable Cox regression.
A total of 187 patients were identified, diagnosed between 1994-2016 (median 2014). At diagnosis, the median age was 65 years (range 41-91); 36% (68/187) of patients were > 70 years old. Follicular lymphoma (FL) was the most common histology [63.4% (97/187); n=54 grade 1-2, n= 31 grade 3A, n=12 unknown grade] followed by 16% (30/187) marginal zone lymphoma, 13% (25/187) Waldenström macroglobulinemia, 12% (22/187) MCL, 2% (3/187) small lymphocytic lymphoma and 5% (10/187) low grade unclassifiable. Median FLIPI score was 2 (range 0-5) and median MIPI score was 5 (range 3-10). 83% (156/187) of patients had Ann Arbor stage III/IV, and 60% (107/178) had extra-nodal disease.
Median follow-up from initiation of BR was 21 months (range 0-45). 81% (152/187) of patients completed six cycles of BR (range 1 to 6). Following treatment, 162 patients were evaluable for response; overall response rate (ORR) was 95% [52% (n=84) CR, 43% (n=69) PR]. Most (n=135) received at least one dose of MR (range 1-12). Of the 67 patients with post MR evaluations, the CR rate increased to 84% (n=56). During follow up, 10% (19/187) of patients relapsed, median time to relapse was 14 months (range 3-31). Of these, 7 had FL (n=5 grade 1-2, n=2 grade 3A). Death (all cause) occurred in 9% (17/187) of patients. Median PFS and OS have not been reached within the follow-up period.
Infections (all grades) were reported in 62% (116/187) of patients with a total of 251 events. Infections were primarily respiratory (55%, 138/251), fever (7%, 17/251) and of the urinary tract (6%, 16/251). Febrile neutropenia events occurred in 3% (5/187) of patients. Non-infectious adverse events (all grades) were reported in 75% (140/187) of patients with a total of 217 events. Fatigue (25%, 54/217), infusion reaction (14%, 31/217), nausea (12%, 25/217) and diarrhea (7%, 16/217) were the most common. Rash was reported in 26% (48/217) of total events.
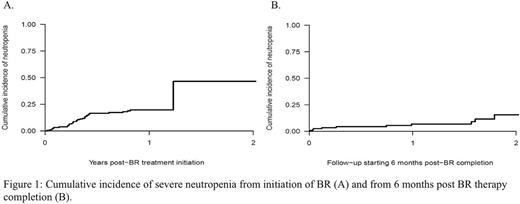
Severe neutropenia occurred in 22% (41/187 patients) of which 5% (n=10) had grade 3 and 17% (n=31) had grade 4. This resulted in a 1-year cumulative incidence of severe neutropenia during BR therapy of 19.7%. Starting after BR completion, there was a 1-year cumulative incidence of neutropenia of 6.9% (Figure 1). Increasing age significantly correlated with risk of neutropenia [p=0.026, HR 1.46, 95% CI (1.05-2.03)]. The 1-year cumulative incidence of respiratory and non-respiratory infections during BR therapy was 35.7% and 39.7%, respectively.
Our real world data of unselected patients (including FL, grade 3A) show that BR is associated with high ORR, similar to the published literature. For those evaluable for post-MR response, CR rate improved compared to post BR assessment. Grade 4 neutropenia was higher than previously reported with older age being a statistically significant risk factor for neutropenia. Risk of neutropenia and infection persisted beyond six months following BR but use of MR could have contributed. Our study show that BR followed by MR is effective for unselected patients with iNHL and transplant ineligible MCL. However, we found higher rates of adverse events than previously reported. Respiratory infection was particularly common and further investigation regarding etiology and benefit of immunizations in this patient population should be explored.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.