Abstract
CD4+CD25+FoxP3+ regulatory T cells (Treg) are broadly suppressive and play a central role in the prevention of chronic graft-versus-host disease (cGVHD) after allogeneic hematopoietic cell transplantation (HCT). Daily subcutaneous (SC) low-dose interleukin-2 (IL-2) at a fixed dose of 1x106 IU/m2/day in adult patients with refractory cGVHD induces selective Treg expansion and leads to clinical responses in 50-60% of evaluable participants (NEJM 2011, Blood 2016). During daily fixed-dose IL-2 therapy, plasma IL-2 levels rose rapidly by week 1, but subsequently decreased over time. The lower IL-2 levels were associated with a decline in Treg proliferation despite daily IL-2 administration (Sci Transl Med 2013), possibly due to IL-2 sequestration via binding to constitutively-expressed high affinity IL-2 receptors on Treg. We reasoned that IL-2 dose escalation at the time of anticipated fall in plasma IL-2 levels would avoid tachyphylaxis and maximize IL-2 induced Treg numbers in vivo.
We conducted a phase 1 trial of individual patient IL-2 dose escalation over 8 weeks in adult and pediatric patients with steroid-refractory cGVHD. Daily SC IL-2 was initiated at 0.67x106 and 0.33x106 IU/m2/day in the adult and pediatric cohorts, respectively. Each participant had dose escalations at weeks 2 and 4 to a maximum dose of 2x106 IU/m2/day in adults and 1x106 IU/m2/day in children. We studied 18 HCT recipients (10 adult: 100% HLA-matched, 100% PBSC grafts, 40% prior aGVHD; 8 pediatric: 87.5% HLA-matched, 87.5% BM grafts, 28.6% prior aGVHD). Median age was 57 years (range, 33-71) in the adult cohort and 12 years (range, 5-22) in the pediatric cohort. The median time from HCT and from cGVHD onset to start of IL-2 treatment was 3 years (range, 1-12.1) and 597 days (range, 234-4336) respectively. Participants had a median of 2 cGVHD organ sites (range, 1-5) and 3 prior cGVHD therapies (range, 1-4) at enrollment. The cohorts did not differ in terms of time since cGVHD onset and number of failed therapies prior to enrollment.
Individually dose-escalated IL-2 was well tolerated in children with 1 participant requiring dose reduction for electrolyte imbalances. In the adult cohort, 1 participant withdrew due to cGVHD progression, 1 required dose reduction for injection site reactions and 2 were unevaluable (1 IL-2 hold >1 month, 1 non-compliance). Seven grade 3 AEs were reported for six participants (electrolyte imbalance, vomiting, infection, increased ALT and soft tissue neoplasm); none relapsed. At week 8, objective cGVHD responses (PR) were seen in 7 of 8 pediatric participants (88%), but in only 1 of 8 evaluable adult participants (13%). cGVHD response sites included skin (n=4), joint/fascia/muscle (n=1), lung (n=4), and GI tract (n=2). Eight pediatric and 4 adult participants with clinical benefit (PR or SD with minor response) continued extended duration IL-2 therapy up to 120 weeks.
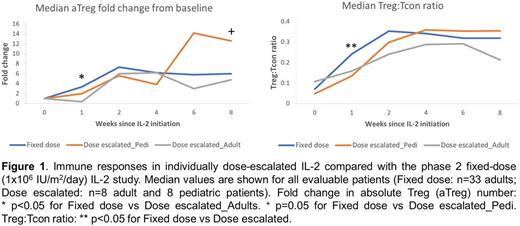
Plasma IL-2 levels were higher in adults at weeks 1 and 2, but immune responses were similar between the 2 cohorts. Dose escalated IL-2 induced a >10-fold increase in median Treg count/µL that peaked at week 6. There were no significant changes in CD4 Tcon, CD8 T cell or B cell count. NK cell count increased >5-fold by week 8. The median Treg:Tcon ratio increased 6-fold from a baseline of 0.05 (Q1-Q3, 0.024-0.114) to a week 5 peak of 0.312 (Q1-Q3, 0.203-0.37). Compared with fixed daily dosing of IL-2 (Blood 2016), the individually dose escalated IL-2 schedule had a slower initial rise in Treg number and Treg:Tcon ratio (Figure 1). This likely reflects the lower starting doses in this study. The maximum IL-2 dose in the adults was twice the previously defined MTD but did not improve Treg expansion. Despite lower IL-2 doses, pediatric patients had a superior clinical response and a trend toward a better immunologic response compared with the adults.
We demonstrate for the first time that low-dose IL-2 is safe in children with advanced steroid-refractory cGVHD and results in a high clinical response rate. A maximum dose of 1x106 IU/m2/day was well tolerated by pediatric patients in the extended duration phase and durably maintained Treg numbers above baseline. Five pediatric patients that continued IL-2 therapy for >20 weeks have reduced or discontinued steroid therapy. In adults, intrapatient dose escalation above fixed-dose MTD was not clinically tolerated and did not result in greater Treg expansion or clinical improvement relative to fixed daily dosing.
Nikiforow: Kite Therapeutics: Membership on an entity's Board of Directors or advisory committees. Armand: Sequenta/Adaptive: Research Funding; Affimed: Research Funding; Roche: Research Funding; Merck & Co., Inc.: Consultancy, Research Funding; Infinity: Consultancy; Genmab: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Otsuka: Research Funding; Pfizer: Consultancy, Research Funding; Tensha: Research Funding; Sigma Tau: Research Funding. Antin: Gentium SpA/Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Koreth: Prometheus Labs: Research Funding; Amgen, Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.