Key Points
ORR was 67% with venetoclax for 36 patients with CLL refractory to or who relapsed after idelalisib; median PFS has not yet been reached.
Common adverse events included mild gastrointestinal events and grade 3/4 cytopenias; no patients experienced tumor lysis syndrome.
Abstract
B-cell receptor pathway inhibitors (BCRis) have transformed treatment of chronic lymphocytic leukemia (CLL); however, the efficacy of therapies for patients whose disease is refractory to/relapses after (R/R) BCRis is unknown. Venetoclax is a selective, orally bioavailable BCL-2 inhibitor with activity in patients with CLL, including those who are heavily pretreated or have 17p deletion. This phase 2 study prospectively evaluated venetoclax in patients with R/R CLL after ibrutinib or idelalisib; here we report on patients who received idelalisib as the last BCRi before enrollment. Venetoclax was initiated at 20 mg daily, followed by intrapatient ramp-up to 400 mg daily. Primary objectives included efficacy (objective response rate [ORR]) and safety of venetoclax. The study enrolled 36 patients who previously received idelalisib (ORR, 67% [24/36]); 2 patients achieved complete remission, and 1 had complete remission with incomplete bone marrow recovery. Median progression-free survival (PFS) has not yet been reached; estimated 12-month PFS was 79%. The most common adverse events (AEs; all grades) were neutropenia (56%), diarrhea (42%), upper respiratory tract infection (39%), thrombocytopenia (36%), nausea (31%), fatigue (28%), cough (22%), rash (22%), and anemia (22%). Grade 3 or 4 AEs were primarily hematologic (neutropenia [50%], thrombocytopenia [25%], and anemia [17%]). No patients experienced tumor lysis syndrome. Venetoclax demonstrated promising clinical activity and favorable tolerability in patients with CLL whose disease progressed during or after idelalisib therapy. This trial was registered at www.clinicaltrials.gov as #NCT02141282.
Introduction
Novel small molecule inhibitors targeting the B-cell receptor signaling pathway (BCRi) are important new treatment options for both untreated and relapsed chronic lymphocytic leukemia (CLL).1,2 The phosphoinositide 3-kinase-δ inhibitor idelalisib combined with rituximab or ofatumumab was approved for relapsed/refractory (R/R) CLL after demonstrating considerable activity in this setting.3,4 Despite the efficacy of idelalisib in combination with anti-CD20 monoclonal antibodies,5-7 approximately one-third of patients with CLL on idelalisib trials progress while receiving therapy.8,9 In addition to progressive disease (PD), treatment-related adverse events (AEs) are a frequent cause of idelalisib treatment discontinuation, and patients who discontinue BCRi therapies typically experience PD shortly thereafter and require additional therapy.7,8 A retrospective study of patients with CLL who discontinued idelalisib-based therapy showed that 52% discontinued because of toxicity.8 Another study found that 27% of patients discontinued idelalisib because of grade 3 or 4 diarrhea or colitis, and 9% because of pneumonia.9 Whether idelalisib is discontinued because of PD or because of toxicity with subsequent PD, the efficacy of various CLL therapies in such patients is unknown.
The optimal treatment of patients with CLL progressing after idelalisib has not been well characterized. Outcomes in patients who discontinued idelalisib treatment early are poor, with 1 retrospective analysis reporting a median overall survival (OS) after idelalisib discontinuation of approximately 2 months (range, 0-10 months).9 Retrospective data suggest that traditional therapies, including chemoimmunotherapy and anti-CD20 monoclonal antibodies, have limited efficacy in this setting.9 Although retrospective data suggest that another agent targeting the BCR pathway, the Bruton’s tyrosine kinase inhibitor ibrutinib, may be active in this population,8 no prospective data on treatment of patients with CLL progressing on idelalisib-based therapies are available. We hypothesized that targeting a pathway other than the BCR pathway would be efficacious in patients progressing on BCRi therapy.
Venetoclax, a selective, orally bioavailable small molecule inhibitor of BCL-2, targets the intrinsic pathway of mitochondrial apoptosis.10 Across clinical studies in R/R CLL, including patients with high-risk disease markers such as chromosome 17p deletion (del[17p]), venetoclax monotherapy induced objective responses in approximately 80% of patients.11,12 Approval of venetoclax in the United States, European Union, and other countries13,14 was based on results from 3 monotherapy studies in R/R CLL, including the study reported here on patients with CLL who progressed after BCRi therapy.11,12,15
Methods
Study design and oversight
This phase 2, open-label, multicenter trial (ClinicalTrials.gov identifier #NCT02141282) initially enrolled patients who had R/R CLL that progressed after receiving prior idelalisib or ibrutinib therapy, with patients assigned to each study group in the main cohort based on the last BCRi received. One group enrolled patients who had received prior idelalisib and the other group enrolled patients who received prior ibrutinib (see supplemental Figure 1, available on the Blood Web site, for trial enrollment information). The study extended enrollment into an expansion cohort to enroll patients who had received prior idelalisib or ibrutinib. This publication only reports on the patients who received idelalisib as their last BCRi before enrollment from both the main cohort and the expansion cohort. Patients treated with venetoclax after receiving ibrutinib as the last BCRi before enrollment are described in a separate publication.16 The primary study objectives were to evaluate the efficacy of venetoclax monotherapy measured by the overall response rate (ORR) and safety, via treatment-emergent AE monitoring and laboratory assessments. Secondary objectives included assessment of duration of response, time to progression, progression-free survival (PFS), and OS. At each participating site, the institutional review board approved the study protocol and amendments. Study activities were conducted in accordance with ethical principles established in the Declaration of Helsinki and the International Conference on Harmonization Guideline for Good Clinical Practice. Written informed consent was obtained from all patients. Patients were recruited from 15 sites across the United States.
Treatment
Venetoclax was administered orally, once daily, beginning no sooner than 7 days after the last dose of previous therapy for the main cohort. Because venetoclax can induce rapid tumor debulking with risk for tumor lysis syndrome (TLS), stepwise intrapatient dose ramp-up, prophylaxis, and monitoring procedures were implemented to mitigate the risk for TLS (see supplemental Appendix for details on prophylaxis and management of TLS). Patients received venetoclax 20 mg daily for 1 week, followed by weekly dose escalation to achieve the target of 400 mg daily by week 5 (supplemental Figure 2). In the expansion cohort, the washout period for prior BCRi was reduced from 7 to 3 days. Furthermore, a compressed dose ramp-up was permitted in patients who had a high tumor burden with clinical signs of progression during screening, to enable administration of the final dose of 400 mg by week 3 (supplemental Figure 3). In addition, dose escalation of venetoclax to 600 mg was allowed for patients who had not achieved a response after the disease assessment at week 12.
Patients
Adults with R/R CLL were eligible if they required therapy according to criteria from the 2008 International Workshop on Chronic Lymphocytic Leukemia (iwCLL).17 Data presented here are from patients enrolled in the main cohort or the expansion cohort who received idelalisib as the last BCRi before trial enrollment.
Complete inclusion and exclusion criteria are provided in the supplemental Appendix. In addition to being refractory to or progressing after discontinuation of idelalisib, key inclusion criteria were an Eastern Cooperative Oncology Group performance score of 2 or less; adequate bone marrow function, defined as an absolute neutrophil count ≥1000/µL irrespective of growth factor support; platelet count ≥30 000/mm3; hemoglobin ≥8 g/dL; and creatinine clearance ≥50 mL/min, using 24-hour measured glomerular filtration rate or estimated by modified Cockcroft-Gault equation. At study entry, all patients were screened for Richter’s transformation by positron emission tomography and were excluded if Richter’s transformation was confirmed on biopsy. Patients with active and uncontrolled autoimmune cytopenias, unresolved toxicity from prior therapy, or a history of allogeneic stem cell transplantation within 1 year of study entry were also excluded.
Assessments
Disease assessments for all patients were performed at screening and at each of the visits. Subsequent responses for patients in the main cohort were assessed by investigators at weeks 8 and 24 and every 12 weeks thereafter, up to 1 year. Patients in the expansion cohort underwent response assessments at weeks 12 and 36. Responses were evaluated by physical exam, laboratory results, computed tomography or magnetic resonance imaging, and bone marrow evaluation according to the 2008 iwCLL criteria.17 Responses were confirmed with a second assessment conducted at least 2 months later. Bone marrow aspiration and biopsy were performed at screening and within 2 months after other criteria for complete remission (CR) were met.
Safety assessments were conducted during the study and up to 30 days posttreatment. Laboratory abnormalities and AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.18
Minimal residual disease (MRD) in peripheral blood and bone marrow was assessed at a central laboratory, using the standardized 6-color flow cytometry protocol published by the ERIC Consortium.19,20 Peripheral blood MRD assessments were scheduled for all patients at week 24. Patients achieving CR or partial remission (PR) required peripheral blood MRD assessments at 12-week intervals thereafter. Bone marrow MRD assessments were planned for all patients at confirmation of a CR and for patients achieving MRD negativity in 2 consecutive peripheral blood samples.
Pharmacokinetic assessments were conducted 8 hours after dosing during the ramp-up period at weeks 1 to 5 and before dosing at weeks 8, 12, 16, and 24. To assess the concentration time profile of venetoclax, predosing plasma concentrations were combined across all visits and categorized by the time relative to the previous dose of venetoclax. Pharmacokinetic results are presented in the supplemental Appendix.
Statistical analyses
The data cutoff date for this publication was June 30, 2017. Efficacy and safety analyses included all patients who received at least 1 dose of venetoclax. Descriptive statistics were calculated, and Kaplan-Meier methods were used for time-to-event analyses. SAS software (SAS Institute, Inc., Cary, NC) was used to generate all statistical summaries. Unless otherwise noted, statistical analyses were 2-sided, with P ≤ .05 considered significant.
The primary efficacy endpoint was the ORR by 2008 iwCLL criteria,17 calculated with a 95% confidence interval based on binomial distribution (supplemental Appendix for information on key secondary efficacy endpoints). Details regarding determination of sample size are provided in the supplemental Appendix.
Results
Patients
A total of 36 patients were enrolled, including 21 in the main cohort and 15 in the expansion cohort. Thirteen patients were considered screen failures, including 1 patient who was excluded because of biopsy-confirmed Richter’s transformation (see supplemental Table 5 for all reasons for screening failures). Enrolled patients had received a median of 3 prior therapies (range, 1-11 prior therapies), including other investigational BCRis, and were receiving prior idelalisib for a median of 9 months (range, 1-45 months; Table 1). While receiving idelalisib, 22 patients had CR or PR as best response to therapy, 7 had stable disease (SD), 2 had no response, and 5 had PD. Reasons for discontinuation of idelalisib included toxicity with subsequent PD (61%; n = 22), PD by iwCLL criteria while receiving idelalisib (36%; n = 13), completion of an idelalisib-containing regimen (3%; n = 1), AEs (19%; n = 7; including recurrent diarrhea, pneumonia, and rash), or other (8%; n = 3 resulting from withdrawal from [n = 1] or closing of an idelalisib clinical trial [n = 1], and 1 patient was deemed a treatment failure [n = 1]). Patients could have more than 1 reason for discontinuation.
Most patients had at least 1 high-risk prognostic feature, including unmutated immunoglobulin heavy chain variable region (IGHV; 88% [22/25]) or high-risk chromosomal abnormalities such as del(17p) (22% [8/36]) and/or TP53 mutation (14% [5/35]). At baseline before dosing, TLS risk as defined in the study protocol (see Table 1) was determined to be low for 10 (28%), medium for 17 (47%), and high for 9 (25%) patients.
Patient disposition
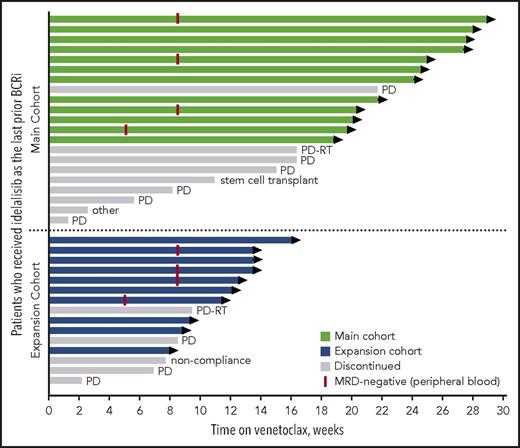
At the time of data cutoff, the median time on venetoclax for all patients was 14 months (range, 1-29 months), with median time receiving venetoclax of 20 months (range, 1-29 months) for the main cohort and 10 months (range, 2-16 months) for the expansion cohort. Twenty-two patients remain active on study (Figure 1). The primary reasons for discontinuation of venetoclax were CLL progression (n = 9), Richter’s transformation (n = 2), elective discontinuation to proceed to allogeneic stem cell transplantation in response (n = 1), patient noncompliance (n = 1), and 1 patient with well-controlled preexisting immune thrombocytopenic purpura (ITP) whose ITP required treatment that did not allow for continuation on study (patient was MRD-negative in peripheral blood). For 11 patients who discontinued venetoclax because of progression (CLL progression, n = 9; Richter’s transformation, n = 2), the median number of prior CLL therapies was 4 (range, 2-9 prior CLL therapies), 88% (7/8 assessed) had unmutated IGHV, 20% (1/5) had del(17p) CLL, and 43% (3/7) had TP53 mutation. Seven of these patients had discontinued prior idelalisib because of PD, 3 discontinued because of toxicity with subsequent progression, and 1 was deemed a treatment failure on idelalisib. Four patients died because of PD.
Patients’ status in the study by treatment group. Swimmers plot depicts the duration of venetoclax therapy, where each bar represents a patient. The primary reasons for discontinuation of venetoclax were CLL progression (indicated as PD), Richter’s transformation (indicated as PD-RT), elective discontinuation to proceed to allogeneic stem cell transplantation in response, patient noncompliance, and 1 patient with well-controlled preexisting ITP whose ITP required treatment that did not allow for continuation on study (indicated as other).
Patients’ status in the study by treatment group. Swimmers plot depicts the duration of venetoclax therapy, where each bar represents a patient. The primary reasons for discontinuation of venetoclax were CLL progression (indicated as PD), Richter’s transformation (indicated as PD-RT), elective discontinuation to proceed to allogeneic stem cell transplantation in response, patient noncompliance, and 1 patient with well-controlled preexisting ITP whose ITP required treatment that did not allow for continuation on study (indicated as other).
Efficacy
The investigator-determined ORR for all patients enrolled was 67% (24/36) (Table 2). Three patients (8%) had a CR or CR with incomplete bone marrow recovery (CRi), and 21 (58%) had PR. The patient with CRi had thrombocytopenia. Median time to first response for all patients was 2.5 months (range, 1.6-8.1 months). Ten patients had SD as best response, and 2 had CLL progression.
Of the 2 patients in the expansion cohort who escalated to a daily dose of 600 mg venetoclax, 1 with SD at week 12 subsequently achieved a PR after escalation to 600 mg, and the second patient escalated to 600 mg because of clinical progression and had no improvement with the higher dose. In the subset of 10 patients who had received ibrutinib for a median of 10 months (range, 2-53 months) as 1 of their treatments before idelalisib, the ORR was 60% (6/10). Among 25 patients who had B symptoms at baseline, 15 (60%) had resolution of symptoms by week 8.15
For 13 patients who discontinued prior idelalisib because of PD, ORR on venetoclax was 46% (6/13), all PR; 5 patients had SD and 2 had CLL progression as best response on venetoclax. For 22 patients who discontinued prior idelalisib because of toxicity, ORR on venetoclax was 82% (18/22), with 1 patient who achieved CR, 1 with CRi, and 16 with PR; 4 patients had SD, and no patients had PD as best response on venetoclax.
Response rates reported by an independent review committee for the 21 patients in the main cohort were similar to those assessed by the investigators. The ORR at 24 weeks by the independent review committee was 62% (13/21), with all 13 patients having achieved a PR, compared with an investigator-assessed ORR of 57% (12/21), with 2 patients in CR, 1 in CRi, and 9 patients with a PR.15
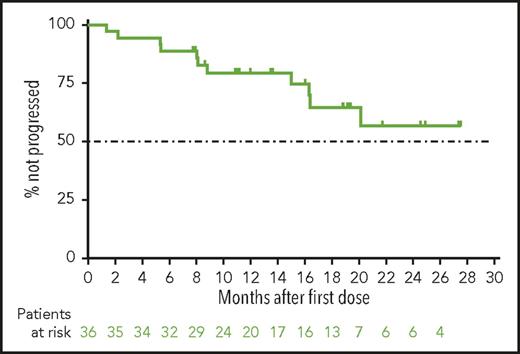
With a median time receiving venetoclax monotherapy of 14 months (20 months for the main cohort and 10 months for expansion cohort), the median PFS (Figure 2), duration of response (not shown), and OS (not shown) have not yet been reached. By investigator assessment, the 12-month PFS rate was an estimated 79% (95% confidence interval, 62%-90%) for all patients. The estimated 12-month OS rate was 94% (95% confidence interval, 78%-99%).
Progression-free survival for patients with CLL progressing after idelalisib who are treated with venetoclax. Shown is the Kaplan-Meier curve for investigator-assessed progression-free survival for all 36 patients from the main and expansion cohorts. Below the curve is the number of patients at risk for the event at each time. Tick marks represent patients censored for each outcome measure.
Progression-free survival for patients with CLL progressing after idelalisib who are treated with venetoclax. Shown is the Kaplan-Meier curve for investigator-assessed progression-free survival for all 36 patients from the main and expansion cohorts. Below the curve is the number of patients at risk for the event at each time. Tick marks represent patients censored for each outcome measure.
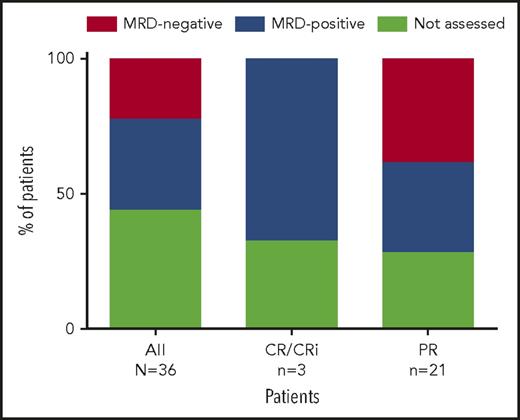
At week 24, 20 patients from both the main and expansion cohorts were assessed for peripheral blood MRD. Eight (40%) of the 17 patients assessed had undetectable MRD in peripheral blood (Figure 3; 8/36 [22%] by intent-to-treat principles), with 2 of these patients (both achieving PR) demonstrating subsequent undetectable MRD in bone marrow based on 10−4 sensitivity (the 6 other patients did not yet have bone marrow assessments). All 8 patients had PR and all continue to receive venetoclax. For the 8 patients who achieved MRD negativity in peripheral blood, the median number of prior therapies was 3 (range, 1-11 therapies); 100% (5/5 assessed) had unmutated IGHV, 14% (1/7) had del(17p) CLL, and 13% (1/8) had TP53 mutation. Seven of these patients discontinued prior idelalisib because of toxicity, and 1 because of PD.
Percentage of patients with MRD negativity in peripheral blood. Eight patients (all PR) assessed were MRD negative in the peripheral blood, with 2 of these patients demonstrating subsequent bone marrow MRD negativity (the 6 other patients have not yet had bone marrow assessments). Shown are the percentage of patients with MRD negativity in peripheral blood based on all 36 patients (intent-to-treat), as well as for 4 patients who achieved CR/CRi as best response and 16 patients who achieved PR as best response.
Percentage of patients with MRD negativity in peripheral blood. Eight patients (all PR) assessed were MRD negative in the peripheral blood, with 2 of these patients demonstrating subsequent bone marrow MRD negativity (the 6 other patients have not yet had bone marrow assessments). Shown are the percentage of patients with MRD negativity in peripheral blood based on all 36 patients (intent-to-treat), as well as for 4 patients who achieved CR/CRi as best response and 16 patients who achieved PR as best response.
Safety
The most common all-grade AEs were neutropenia (56%), diarrhea (42%), upper respiratory tract infection (39%), thrombocytopenia (36%), nausea (31%), fatigue (28%), cough (22%), rash (22%), and anemia (22%; Table 3). Grade 3 or 4 AEs were primarily hematologic, including neutropenia (50%), thrombocytopenia (25%), and anemia (17%), as well as hypokalemia (11%). Fourteen (39%) patients received granulocyte-stimulating growth factor during the study for neutropenia with or without infection. No patients had febrile neutropenia. Serious AEs were infrequent and included pneumonia (n = 2) and cholecystitis (n = 2; Table 3). There were no deaths attributed to adverse events. No cases of clinical TLS were reported. Three patients whose laboratory abnormalities met criteria for laboratory TLS per Howard et al21 were identified as having confounding factors, including 1 patient with obstructive uropathy from bulky retroperitoneal adenopathy secondary to CLL, a patient with spontaneous TLS before venetoclax, and a patient who had pseudohyperkalemia confirmed by whole-blood potassium levels. Eight patients had dose interruptions, most commonly for diarrhea (n = 5). Eight patients reduced their venetoclax dose, some for more than 1 reason, most commonly because of neutropenia (n = 6), thrombocytopenia (n = 5), fatigue (n = 5), or anemia (n = 4). One patient in the expansion cohort who was scheduled to receive compressed ramp-up because of rapidly progressive CLL developed pneumonia at the 200-mg dose and required dose interruption for 1 week and then resumed dosing to reach the final dose of 400 mg by week 4. This patient discontinued study because of PD by day 129.
Discussion
BCRi therapies have transformed the management of both previously untreated and relapsed CLL. Little is known about treatment of patients who have progressed on these new drugs, and most patients with R/R CLL who discontinue idelalisib early have limited options with poor outcomes.8,9 For example, 1 retrospective report showed that patients with R/R CLL who discontinued idelalisib had a median OS of 64 days from the time of treatment discontinuation.9 Here, we report the results of the first prospective study of patients with R/R CLL who were previously treated with idelalisib and who progressed.
The response rate and durability for venetoclax in this population are promising, with 67% ORR and median PFS and OS not yet reached after a median of 14 months of follow-up. Most nonresponders had SD (n = 10). The low number of CRs reported at the time of analysis may be a result of the follow-up time, particularly for patients in the expansion cohort, as other clinical studies with venetoclax report CR occurring after 1 year on therapy.12 Patients with prior ibrutinib exposure who had progressed on idelalisib as their most recent therapy before study entry had similar efficacy results.
Most patients (60%) with MRD-negative PR had residual adenopathy in 1 or 2 nodes, and others had mild residual splenomegaly. All patients with MRD negativity in blood continue receiving active treatment. Even though the number of patients reported here is small, it is notable that some patients did achieve MRD negativity in the peripheral blood and marrow with a single targeted agent, given this highly refractory population. A recent study showed no statistical difference in PFS or OS after chemoimmunotherapy among patients in PR with undetectable MRD, including those with residual splenomegaly, marrow involvement, or 1 or more involved site compared with patients who were in CR with undetectable MRD in blood.22
As described in a recent separate publication, the efficacy and safety of venetoclax monotherapy was similar for patients who had progressed after prior ibrutinib.16 The ORR for these patients was 65% (59/91), and median PFS was 24.7 months. The estimated 12-month PFS and OS rates on venetoclax for patients who had received ibrutinib as their last BCRi before study enrollment were 75% and 91%, respectively. In addition, 10 patients who received idelalisib followed by ibrutinib during their course of treatment of CLL before study enrollment had an ORR on venetoclax of 70% (7/10).
The safety profile for venetoclax postidelalisib is consistent with previous reports of venetoclax monotherapy in R/R CLL,11,12 as well as in patients who received venetoclax postibrutinib.16 All patients followed the 5-step dose ramp-up (1 patient received venetoclax over a compressed 3-week schedule), and by implementing risk-based prophylactic measures and close monitoring, TLS was not observed, despite many of these patients having rapidly progressive CLL after discontinuing idelalisib. Close attention was paid to TLS prophylaxis, and management on this study as is now outlined in the venetoclax prescribing label.13 As such, clinicians starting venetoclax in patients who are progressing on idelalisib outside the clinical trial setting should closely follow the label guidelines to mitigate the risk for TLS. On the basis of the experience in this trial, we suggest that patients discontinue idelalisib no more than 3 days before starting to receive venetoclax. Although cytopenias were common in this population of heavily pretreated patients, they did not lead to discontinuation of venetoclax. Despite an incidence of grade 3 or higher neutropenia in 50% of patients, the rates of infection were modest, with no episodes of febrile neutropenia, likely at least in part because of the common use of concomitant growth factor support on trial.
In summary, venetoclax monotherapy is active and well-tolerated in patients with CLL progression after therapy with idelalisib, including a significant number of patients who also received prior therapy with ibrutinib. These results from the first prospective trial in this high-risk population provide evidence that venetoclax should be considered as a treatment option for such patients.
Presented in abstract form at the 58th Annual Meeting of the American Society of Hematology, San Diego, CA, 1 December 2016.
Presented in abstract form at the 52nd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, 6 June 2016.
Presented in abstract form at the 57th Annual Meeting of the American Society of Hematology, Orlando, FL, 5 December 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
AbbVie and the authors thank the patients who participated in this clinical trial and their families, as well as study investigators, coordinators, and support staff.
Venetoclax is being developed in collaboration between AbbVie and Genentech. AbbVie and Genentech provided financial support for the study and participated in the design, study conduct, and analysis and interpretation of data, as well as the writing, review, and approval of the publication. Medical writing support was provided by Sharanya Ford and Deborah Eng, ELS, after generation of the initial draft; both are employees of AbbVie.
Authorship
Contribution: S.C. and J.A.J. designed the study, collected data, analyzed and interpreted data, and wrote the manuscript; M.C., R.R.F., H.E., L.H., W.G.W., and M.S.D. collected data, analyzed and interpreted data, and wrote the manuscript; B.C., L.Z., S.A., T.W., and M.V. analyzed and interpreted data and wrote the manuscript; and. R.A.H. and J.P. designed the study, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: S.C. is an advisory board member for AbbVie, Pharmacyclics, Gilead, Janssen, and Genentech, and receives institutional research funding from AbbVie, Pharmacyclics, Gilead, and Janssen. M.C. is on the advisory board/performs consultancy for AbbVie and Genentech; receives institutional research funding from AbbVie and PCYC; and is on the speakers bureau for Gilead, AbbVie, PCYC, and Genentech. R.R.F. is a consultant for AbbVie, Pharmacyclics, Janssen, Gilead, and Genentech. H.E. is a consultant for AbbVie, Gilead, and Genentech; an advisory board member for AbbVie, Gilead, and Genentech; and speaker for AbbVie, Gilead, and Genentech. L.H. receives institutional research funding from AbbVie and Pharmacyclics. J.A.J. is an advisory board member for Genentech, AbbVie, and Pharmacyclics, and receives institutional research funding from AbbVie, Pharmacyclics, and Genentech. W.G.W. receives research funding from AbbVie and Genentech and is a consultant and on the speaker bureau for Genentech. M.S.D. is an advisory board member for Genentech, TG Therapeutics, Gilead, and Incyte; receives institutional research funding from Genentech, Pharmacyclics, TG Therapeutics, and Infinity; and is a consultant for Genentech, AbbVie, Pharmacyclics, Janssen, and Merck. B.C., L.Z., S.A., T.W., M.V., R.A.H., and J.P. are AbbVie employees and may own stock.
Correspondence: Steven Coutre, Department of Medicine/Hematology, 875 Blake Wilbur Dr, Stanford, CA 94305, e-mail: coutre@stanford.edu.