Key Points
The majority of ineligible patients had missing documentation or laboratory values outside of the protocols’ defined time frames.
Safety and efficacy outcomes between ineligible and eligible patients enrolled on SWOG leukemia studies were comparable.
Abstract
Patients may be deemed ineligible for a clinical trial for reasons that do not directly impact efficacy or safety. We identified reasons for ineligibility and compared outcomes of ineligible with eligible patients treated on Southwest Oncology Group (SWOG) Leukemia Committee protocols. Patients enrolled in SWOG phase 2, 2/3, or 3 protocols open since 2005 were analyzed for eligibility status, reasons for ineligibility, baseline characteristics, Eastern Cooperative Oncology Group (ECOG) performance status (PS), serious adverse events (SAEs), complete remission (CR) status, and overall survival. A total of 2361 patients were enrolled in the 13 included studies. Of these, 247 (10%) were deemed ineligible; 78 were excluded from analyses, and 169 were included. Of the 169 included in analyses, 60% (101/169) were excluded due to missing baseline documentation. Baseline characteristics comparing ineligible to eligible patients were similar, with the exception of ECOG PS for S0325 (P = .02) and S0530 (P = .002). In multivariable analyses, neither the proportion of patients with ECOG PS ≥ 2 (P = .12) nor the rate of grade 5 SAEs (P = .62) differed between groups. There was no difference in survival between eligible and ineligible patients (P = .25), and CR rates were similar, with the exception of S0325 (P < .001) and S0703 (P = .004). The findings of this study suggest that nonessential eligibility criteria can be less restrictive, thus expanding patient enrollment and avoiding protocol deviations. The clinical trials included in this study were registered at www.clincialtrials.gov as #NCT00085709, #NCT00066794, #NCT00070499, #NCT00109837, #NCT00093418, #NCT00492856, #NCT00337168, #NCT00352365, #NCT00658814, #NCT00792948, #NCT00945815, #NCT00840177, and #NCT01522976.
Introduction
Despite the concerted effort to improve recruitment to cancer clinical trials, enrollment continues to be a challenge, with only 3% to 5% of adult cancer patients in the United States accrued to such studies.1 As a result, almost 20% of publicly funded cancer clinical trials closed because of insufficient accrual.2 Enrollment challenges have led to delays in the time it takes to test the effectiveness of new therapies, while unbalanced accrual of specific patient populations diminish the generalizability of the trials’ results.3
Variables contributing to limited cancer clinical trial access and generalizability of results include overly restrictive eligibility criteria, both in solid tumors4-6 and in hematologic malignancies.7 Thus, patients may be deemed ineligible for reasons unrelated to potential drug efficacy or safety. One single-institution study deliberately enrolled myelodysplastic syndromes (MDS) patients ineligible for other studies to a trial randomizing subjects to azacitidine monotherapy or azacitidine combined with vorinostat and found response rates and toxicities to be acceptable and similar to studies enrolling traditionally eligible patients.8 Reasons for ineligibility to hematological malignancy protocols, specifically within a leukemia population, and comparisons of toxicities and outcomes for eligible and ineligible patients treated on those protocols, has not previously been explored. We identified and categorized reasons for ineligibility and compared outcomes of ineligible patients with eligible patients treated on Southwest Oncology Group (SWOG) Leukemia Committee clinical trials.
Methods
Patients had a diagnosis of acute myeloid leukemia (AML), acute promyelocytic leukemia (APL), acute lymphocytic leukemia (ALL), MDS, chronic lymphocytic leukemia (CLL), or chronic myeloid leukemia (CML) and were enrolled and treated in 13 SWOG Leukemia Committee phase 2, 2/3, or 3 protocols between 2005 and 2015 (S0106, S0301, S0325, S0333, S0432, S0521, S0530, S0605, S0703, S0805, S0910, S0919, and S1117) (Table 1). Protocol design and eligibility criteria have been described previously.9-20 Diagnoses were centrally confirmed and per World Health Organization criteria.21
All patients registered on the 13 SWOG protocols were initially identified and categorized according to their eligibility status. The eligible patients fully met the inclusion/exclusion criteria, while the ineligible patients were initially registered as eligible despite having violated at least 1 criterion. The inadvertent enrollment of ineligible patients was only identified retrospectively by SWOG during central review, site audits, and/or database inquiries.
SWOG requires the same data submission for all registered patients; these studies have treatment, adverse event, and response data on all patients who receive protocol therapy. Long-term outcome data, such as overall survival, is collected for all patients, regardless if they are eligible or not. We categorized the eligible and ineligible patients across the 13 protocols into 3 groups: (1) ineligible (excluded from the SWOG studies’ analyses), (2) ineligible (treated on study and included in the SWOG studies’ analyses), or (3) eligible and included in the SWOG studies’ analyses. The majority of patients in group 1 were identified as ineligible after they were enrolled (eg, registered as eligible) but before they received treatment; therefore, the data collected for these patients was limited to baseline characteristics. Patients categorized in group 2 actively participated on study and had treatment, adverse event, and response (including duration) data collected throughout their participation. Thus, our analyses compare group 2 and group 3, as outcome data were limited for patients excluded from studies’ analyses (group 1).
Reasons for ineligibility were categorized and summarized descriptively. All analyses used primary patient data. Ineligible patients were analyzed per protocol; for single-arm studies, only patients who received any protocol therapy were reported in trial publications and are included in the following analyses. In randomized studies, patients were analyzed using intent-to-treat methodology and are included in the following analyses regardless of treatment received.
We evaluated eligibility status; baseline characteristics (age, sex, white blood cells, hemoglobin, platelets, bone marrow blasts, peripheral blasts, performance status [PS]); immunophenotype; disease status at the time of study entry; prior treatment; Eastern Cooperative Oncology Group (ECOG) PS; blood/bone marrow, cardiac, hepatic, renal, and thoracic grade 3 to 5 adverse events/serious adverse events (SAEs) as per the Common Terminology Criteria for Adverse Events; overall survival (OS); and complete remission (CR). Response was defined per contemporary international working group accepted criteria specific to each disease and as previously reported for each study. OS was measured from date of study enrollment to date of death due to any cause and was censored at the date of last contact for patients last known to be alive.
Baseline characteristics for ineligible vs eligible patients were compared within each protocol using Fisher’s exact and Wilcoxon rank sum tests. Outcomes of the analyzed ineligible patients (ineligible and included in the studies’ analyses) were compared with the eligible patients (eligible and included in the studies’ analyses) using multivariable logistic regression (ECOG PS, CR, and SAEs) and Cox regression (OS) analyses, controlling for age, sex, study design (randomized vs single arm), and disease type (relapse/refractory vs de novo). When analyzing pooled data across studies, generalized estimating equations and random effects models were used to account for within-study correlation.
CR rates and adverse event rates varied significantly across protocols, and analyses across protocols were confounded by varying rates of ineligibility and CR across protocols. We therefore used Fisher’s exact (univariate) test and logistic regression (multivariable controlling for age, sex, and PS [except for studies S0333 and S0530, which had too few patients with PS ≥2 to include that variable in the models]) to analyze these outcomes within each study that had at least 5 ineligible patients.
Significance was defined as 2-sided α ≤ .05. All statistical analyses were carried out using R. This study adheres to the ethics defined by the Declaration of Helsinki with each protocol approved by individual institution and National Cancer Institute review boards.
Results
Characteristics of ineligible patients
A total of 2361 patients were enrolled in the 13 included studies. Of these, 10 were phase 2 (77%), 2 (15%) were phase 3, and 1 (8%) was phase 2/3. The majority of studies included patients with de novo disease (10/13; 77%): AML (n = 5), CML (n = 1), ALL (n = 2), APL (n = 1), and MDS/chronic myelomonocytic leukemia (n = 1). The remainder (3/13; 23%) enrolled previously treated patients (ALL, n = 2; AML, n = 1) (Table 1).
Across the 13 studies, 247 patients (10%) were deemed ineligible; 78 were excluded from trial analyses (Table 2), and 169 were included (Table 3). Of the 78 patients excluded from analyses, 73% (57/78) did not have the disease of interest, 19% (11/57) of whom were AML patients with myeloblasts just below the percentage required for diagnosis (eg, <20% myeloblasts) (Table 4). The remaining 21 patients were ineligible for the following reasons: abnormal hematologic laboratory value (6/78; 8%), abnormal hepatic laboratory value (3/78; 4%), abnormal cardiac function (2/78; 2%), diagnosis incomplete (3/78; 4%), age (1/78; 1%), and other (6/78; 8%) (Table 2).
The primary reasons for ineligibility among the 169 patients treated on studies and included in analyses were missing baseline documentation (101/169; 60%) or acceptable laboratory values outside of the protocol-defined time window (27/169; 16%) or out of window bone marrow biopsy (15/169; 9%) (Table 3). The few patients ineligible due to disease (4/169; 2%) were enrolled on S0325 (CML in chronic phase); although they likely had the disease of interest, SWOG was unable to confirm their diagnosis because of missing documentation (eg, quantification of hematopoietic subtypes) on their pathology reports.
Of those subjects excluded due to the timing of their bone marrow biopsy (eg, 14-day bone marrow biopsy window), the median number of days out of window was 4.5 (range, 1-120) days. All patients ineligible due to out of window laboratory values did not meet the S0432 (phase 2 studies of 2 different schedules and 2 different doses of the farnesyl transferase inhibitor R115777 (tipifarnib, Zarnestra, NSC-702818) for previously untreated acute myeloid leukemia (AML) in patients of age 70 or older)12 criterion requiring the white blood cell count to be ≤30 × 109/L within 1 day of registration. The most common missing baseline documentation items leading to ineligibility were specimens required for correlative studies (59/101; 58%), enumeration of bone marrow promyelocytes (19/101; 19%) in non-APL, AML trials, pathology report not submitted (9/101; 9%), and inadequate bone marrow/dry tap (7/101; 7%) (supplemental Table 1, available on the Blood Web site).
Comparing characteristics of ineligible to eligible patients
Comparing ineligible to eligible patients, baseline characteristics, with the exception of ECOG PS for studies S0325 (P = .02) and S0530 (P = .002), were similar (supplemental Tables 2-14). In multivariable analyses, neither the proportion of patients with ECOG PS 2 or higher (odds ratio [OR], 0.60; 95% confidence interval [CI], 0.32, 1.15; P = .12) nor the rate of grade 5 SAEs (OR, 0.69; 95% CI, 0.17, 2.99; P = .62) differed between groups. For grade 3 to 5 adverse events subcategorized by body system (blood/bone marrow, cardiac, hepatobiliary, renal, thoracic), there were only 3 significant differences between ineligible and eligible patients: grade 4 blood/marrow disorders were significantly lower among ineligible patients in S0333 and S0805 (S0333: 75% vs 30%, P = .01; S0805: 79% vs 33%, P = .01) but significantly higher in S1117 (57% vs 76%, P = .048) (supplemental Tables 15-27).
Comparing outcomes of ineligible to eligible patients
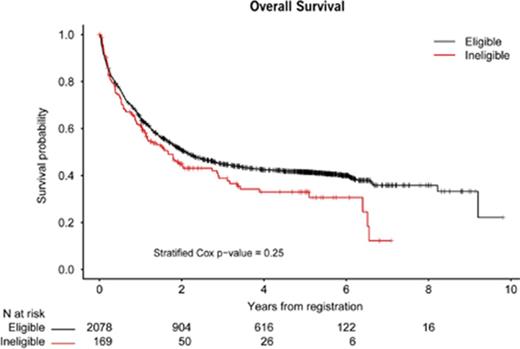
In univariate and multivariable analysis, CR rates were significantly lower among ineligible patients compared with eligible patients in S0325 (67% vs 92%, P < .001), while CR rates were significantly higher among ineligible patients in S0703 (75% vs 23%, P = .004). There were no significant differences in CR rates among ineligible and eligible patients among the 6 other protocols: S0106 (50% vs 70%, P = .10), S0333 (20% vs 55%, P = .09), S0432 (3% vs 6%, P = .71), S0530 (11% vs 7%, P = 1.00), S0805 (80% vs 87%, P = .62), and S1117 (22% vs 22%, P = 1.00) (Table 5). There was also no difference in OS between ineligible and eligible patients analyzed across all of the protocols (P = .25) (Figure 1); the result remained not significant when excluding disease trials for which the majority of patients would be expected to have prolonged survival (S0325 [CML] and S0521 [APL]; P = .09). Furthermore, in a multivariable model of OS among all patients, eligibility status was not significantly associated with OS when controlling for age, sex, study design, and disease (hazard ratio, 1.12; 95% CI, 0.89, 1.40; P = .37) (Table 6).
Discussion
A minority of the adult cancer population is enrolled in clinical trials, and it has become a national imperative to change this to meet patient demands, maximize scientific rigor, improve study efficiency, and broaden study generalizability. This will require a 2-pronged approach, focusing on patient-level barriers such as knowledge and perception, and organizational level obstacles, such as overly restrictive eligibility criteria.
The first step in modifying these obstacles is to identify why patients were excluded from cancer trials. We found that the majority of patients who registered but were ineligible for SWOG Leukemia Committee protocols had either missing documentation or laboratory values outside of a protocol’s specified time frame. All of these patients, with the exception of one subject (bone marrow 120 days out of protocol-defined window), had these tests performed within a week of the protocol-defined window. From a clinical perspective, a week is unlikely to meaningfully impact most patients’ diagnosis, suggesting these time frames (eg, a 14-day bone marrow biopsy window) may be inappropriately narrow. On the contrary, a week may be clinically appropriate for specific patient populations, particularly those with a clinical status that is rapidly changing (eg, acute promyelocytic leukemia) and for laboratory values that could impact drug efficacy or safety. Therefore, these time frames should be revised to reflect the appropriate range of days required to adequately assess the patient population’s clinical fitness. In some cases, the time frame for screening tests should increase while in others this may need to decrease.
Furthermore, 24% (10/41) of the subjects deemed ineligible because of their AML diagnosis had between 10% and 19% blasts (Table 4), which is just below the blast requisite as per the World Health Organization classification system (≥20%). Although it is controversial whether a difference in survival outcomes exists in patients with 20% to 29% or 10% to 19% blasts,22 the current International Prognostic Scoring System-Revised for MDS23 suggests that patients who cross a threshold of ≥10% blasts have a similar survival, whether the blasts are at a level of 11% or 29%.
Patients deemed ineligible because of missing baseline specimen samples unrelated to diagnosis confirmation (59/247; 24%) also may have been unreasonably excluded, as the specimens were not required to measure the primary clinical objectives of any of these studies. Newer SWOG protocols recognize the limitations of such criteria, requesting baseline samples are submitted if available, which ensures patients are not penalized solely because of the specimen submission criterion. Given SWOG’s revised eligibility requirements regarding baseline samples, we performed additional subgroup analyses to determine whether the 59 subjects who were ineligible due to missing baseline samples diluted our results. Comparing the ineligible patients due to missing documentation (labeling the 59 with missing baseline specimens as eligible) to the eligible patients did not change our results (OS [P = .48], CR [P = .31], toxicity [grade 5 (P = .57) or grade 4 (P = .17)]), suggesting those patients ineligible due to other missing documentation may perform similarly to the eligible patients.
The next step in modifying organization obstacles to cancer trial enrollment is to assess whether ineligible patients actually treated on such trials experienced more toxicities to treatment and thus should have been excluded. We and others have previously shown that eligibility criteria do not reflect anticipated or realized drug toxicities.7 While liberalizing criteria can improve applicability to “real-world” patients, there is the potential that previously ineligible patients could be harmed by experimental therapies. As the majority of the ineligible patients were excluded because of administrative reasons, it is not surprising that there were no significant differences between ineligible and eligible patients with respect to ECOG PS, grades 3 to 5 cardiac, hepatic, renal, or thoracic adverse events, and OS, with a mixed picture for grade 3 or 4 blood/marrow disorders.
Given these results, we expect multivariable analysis aiming to identify if eligibility is a risk factor for adverse events, controlling for ineligibility reason (eg, bone marrow out of window, missing documentation, or abnormal laboratory value), age, sex, PS, study design, and disease type would have yielded comparable results; specifically demonstrating all ineligible patients, despite their reason for exclusion, would have performed similarly to the eligible patents. Furthermore, we anticipate no differences in grade 3 to 5 adverse events between patients with high (ECOG PS ≥2) and low (ECOG PS <2) PS would have been revealed. Unfortunately, the results from this multivariable analysis are not reported because the small sample size made the model unstable.
The final step in modifying eligibility criteria is to determine whether enrolling previously ineligible patients would compromise efficacy. The majority of studies also demonstrated there were no significant differences in complete response rates between ineligible and eligible patients, with one (S0703) actually demonstrating, potentially because of prolonged time to treat in older AML patients,24 a higher CR rate for ineligible patients than eligible patients. More importantly, OS did not differ between the 2 groups.
This study reports on a decade of experience in one US Cooperative Group’s leukemia studies. Thus, the population cannot be used to make inferences about the general population of patients excluded from these studies (eg, all patients who were not registered because they were ineligible due to an abnormal test result or laboratory value/normal test result or laboratory value resulted outside of the protocol-defined time frame). Consequently, it likely underestimates the true numbers of patients who could have been eligible for these protocols had the eligibility criteria been expanded slightly (eg, allowing 1 extra week for bone marrow assessment) and does not reflect the outcomes realized by the general population of leukemia patients. Future studies should determine whether outcomes are comparable between real-world populations representing eligible and ineligible patients. Although this limitation precludes the broad generalization of our results, to our knowledge, this is the first study to evaluate how predominately administrative-related criteria may limit patients’ access to clinical trials and how liberalizing these criteria would do little to impact adverse events or outcome.
Overall, our results indicate patients on these SWOG trials who failed to meet nonessential eligibility criteria, specifically those associated with missing documentation, such as a baseline specimen sample, and out of window laboratory values or bone marrow biopsies, had similar outcomes to eligible patients. Our findings suggest, across the 13 analyzed studies, the modification of these criteria may have led to a 10% increase in accrual. Thus, patient enrollment to such studies could be enhanced through simple revision of specific eligibility criteria, such as removing sample collection mandates if correlative testing is not a primary end point, extending the typical time frame for laboratory tests and bone marrow biopsies, and even broadening diagnostic criteria when they make clinical sense.
The application of our findings extends beyond SWOG studies. Industry-sponsored studies may also include patients initially deemed ineligible for reasons that are not clinically significant, granting those patients waivers. Although SWOG does not permit eligibility waivers,25 the outcomes of patients not meeting strict eligibility criteria but still treated on SWOG studies, as would be the case with patients receiving waivers by industry sponsors for the same ineligibility reasons (eg, tests performed outside of the protocol defined time frame/clinically insignificant abnormal laboratory values), are presumably comparable to those of ineligible patients waived onto industry-sponsored clinical trials. If similar clinically insignificant criteria are therefore modified by 2 major sponsors of clinical trials in the United States, patients’ access to novel treatments could increase dramatically, ultimately improving the generalizability of these trials’ results, minimizing protocol violations, and accelerating the time it takes to bring new products to market.
Presented in poster form at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 5 December 2016.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute, National Institutes of Health (grants CA180888, CA180819, CA180858, CA180816, CA04919, and CA46282). M.A.S. is funded, in part, by the Edward P. Evans Foundation.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: A.S. designed the research, collected the data, analyzed the data, and wrote the paper; M.A.S. designed the research, collected the data, and wrote the paper; M.O. collected the data, analyzed the data, and wrote the manuscript; H.P.E., T.R.C., J.P.R., S.C., A.A., S.N., F.R., and M.A.S. designed the clinical trials included in the analyses, collected the data for the clinical trials included in the analyses, and wrote the paper; and S.M. wrote the paper.
Conflict-of-interest disclosure: M.A.S. serves on Celgene’s Advisory Board. A.A. provides consulting services for Pfizer. S.M. provides consulting services for, receives research funding and honoraria from, and serves on the speakers bureau for Novartis, Takeda, Celgene, Pfizer, and Bristol Myers Squibb. S.C. provides consulting services for and receives research funding from Novartis and Celgene. H.P.E. serves on the speakers bureau for Celgene, Incyte, Jazz, and Novartis; provides consulting services for Amgen, Celgene, Daiichi Sankyo, Jazz, ImmunoGen, Incyte, MacroGen, Novartis, Ono, Pfizer, Seattle Genetics, Sunesis, and Millennium/Takeda; receives research funding from Agios, Amgen, Juno, Astellas, Celator, Daiichi Sankyo, ImmunoGen, Janssen, Millennium/Takeda, and Seattle Genetics; is the Chair of the Data and Safety Monitoring Committee for Glycomimetics; and is the Chair of Celgene’s Scientific Steering Committee. The remaining authors declare no competing financial interests.
Correspondence: Mikkael A. Sekeres, Leukemia Program, Cleveland Clinic Taussig Cancer Institute, Cleveland, OH 44195; e-mail: sekerem@ccf.org.