Key Points
Analysis of full VKORC1 reduction of vitamin K epoxide vs the individual reactions shows that warfarin uncouples the 2 reactions.
A mutant becomes more active than wild-type VKORC1 only in the presence of warfarin, revealing a novel mechanism for warfarin resistance.
Abstract
The anticoagulant warfarin inhibits the vitamin K oxidoreductase (VKORC1), which generates vitamin K hydroquinone (KH2) required for the carboxylation and consequent activation of vitamin K–dependent (VKD) proteins. VKORC1 produces KH2 in 2 reactions: reduction of vitamin K epoxide (KO) to quinone (K), and then KH2. Our dissection of full reduction vs the individual reactions revealed a surprising mechanism of warfarin inhibition. Warfarin inhibition of KO to K reduction and carboxylation that requires full reduction were compared in wild-type VKORC1 or mutants (Y139H, Y139F) that cause warfarin resistance. Carboxylation was much more strongly inhibited (∼400-fold) than KO reduction (two- to threefold). The K to KH2 reaction was analyzed using low K concentrations that result from inhibition of KO to K. Carboxylation that required only K to KH2 reduction was inhibited much less than observed with the KO substrate that requires full VKORC1 reduction (eg, 2.5-fold vs 70-fold, respectively, in cells expressing wild-type VKORC1 and factor IX). The results indicate that warfarin uncouples the 2 reactions that fully reduce KO. Uncoupling was revealed because a second activity, a warfarin-resistant quinone reductase, was not present. In contrast, 293 cells expressing factor IX and this reductase activity showed much less inhibition of carboxylation. This activity therefore appears to cooperate with VKORC1 to accomplish full KO reduction. Cooperation during warfarin therapy would have significant consequences, as VKD proteins function in numerous physiologies in many tissues, but may be poorly carboxylated and dysfunctional if the second activity is not ubiquitously expressed similar to VKORC1.
Introduction
Carboxylation of vitamin K–dependent (VKD) proteins is required for their functions in diverse physiologies. Virtually every tissue expresses at least 1 of the 16 known VKD proteins, which have functions that include hemostasis, calcium homeostasis, signal transduction, growth control, and apoptosis.1 Carboxylation uses vitamin K cycling between the γ-glutamyl carboxylase and vitamin K oxidoreductase (VKORC1), which both reside in the endoplasmic reticulum and mediate VKD protein modification during secretion. The carboxylase uses oxygenation of vitamin K hydroquinone (KH2) to vitamin K epoxide (KO) to convert Glus to carboxylated Glus (Glas). The KO product is then recycled by VKORC1 (Figure 1A). Mutations in the carboxylase and VKORC1 can impair carboxylation and decrease VKD clotting factor activities to cause severe bleeding,2,3 and a lethal hemorrhagic response is observed in VKORC1 and carboxylase knockout mice.4,5
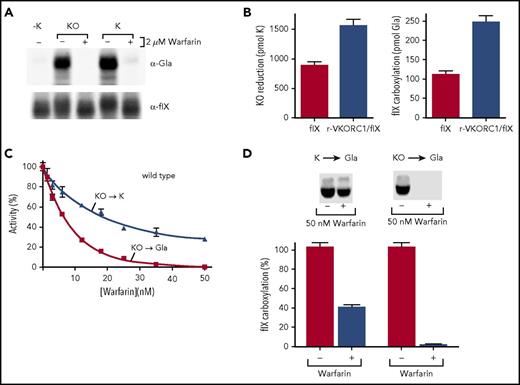
The Y139F VKORC1 mutant fully reduces KO to KH2to drive carboxylation. (A) Cycling between oxidized (KO) and reduced (KH2) vitamin K drives VKD protein carboxylation by the γ-glutamyl carboxylase. VKORC1 fully reduces KO to the quinone intermediate (K) and then to KH2; an unknown warfarin-resistant quinone reductase can also perform the second reaction. (B) Purified human VKORC1 bearing the Y139F mutation was tested for KO reduction to KH2 by performing all steps under nitrogen to block KH2 oxidation. (C) Microsomes containing only the carboxylase (Carb) or also expressing wt or Y139F VKORC1 were tested for KO or K supported carboxylation, as assessed by [14C]-CO2 incorporation into the peptide FLEEL. (D) The microsomes in panel C were also tested for KH2-supported carboxylation. (E) Insect cells containing tagged (Y139Fflag) and untagged Y139F or only the individual forms were subjected to immunopurification with anti-FLAG antibody, followed by western analysis using antibody against VKORC1, as before.22 Mock indicates uninfected cells.
The Y139F VKORC1 mutant fully reduces KO to KH2to drive carboxylation. (A) Cycling between oxidized (KO) and reduced (KH2) vitamin K drives VKD protein carboxylation by the γ-glutamyl carboxylase. VKORC1 fully reduces KO to the quinone intermediate (K) and then to KH2; an unknown warfarin-resistant quinone reductase can also perform the second reaction. (B) Purified human VKORC1 bearing the Y139F mutation was tested for KO reduction to KH2 by performing all steps under nitrogen to block KH2 oxidation. (C) Microsomes containing only the carboxylase (Carb) or also expressing wt or Y139F VKORC1 were tested for KO or K supported carboxylation, as assessed by [14C]-CO2 incorporation into the peptide FLEEL. (D) The microsomes in panel C were also tested for KH2-supported carboxylation. (E) Insect cells containing tagged (Y139Fflag) and untagged Y139F or only the individual forms were subjected to immunopurification with anti-FLAG antibody, followed by western analysis using antibody against VKORC1, as before.22 Mock indicates uninfected cells.
KO reduction to the KH2 form required for carboxylation occurs in 2 reactions that involve a vitamin K quinone intermediate (Figure 1A). We showed that VKORC1 efficiently performs both reductive steps.6 A VKORC1 dimer was suggested by combined immunoprecipitation and western analysis6 and by a split-ubiquitin screen.7 Importantly, an inactive VKORC1 mutant lacking active site thiols showed a dominant negative effect on VKORC1-supported carboxylation,6 indicating the importance of a VKORC1 dimer to efficient KO to KH2 reduction.
Although VKORC1 and the paralog VKORC1L1 are the only enzymes known to reduce KO,8,9 K reduction by at least 1 additional enzyme has been indicated by clinical studies on patients undergoing warfarin therapy (Figure 1A). This anticoagulant targets VKORC1 to dampen hemostasis and prevent thrombotic complications.10 Patients overdosed with warfarin are given vitamin K as an antidote, which rescues hemostasis because a warfarin-resistant quinone reductase is present in liver,11 where the VKD clotting factors are synthesized. The identity of the antidotal reductase is unknown.
The mechanism by which warfarin inhibits VKORC1 is poorly understood. For example, it is unknown why mutations in VKORC1 cause warfarin resistance (ie, the need for higher warfarin doses to manage hemostasis). Also unknown is the effect of warfarin on full reduction of KO to KH2. Biochemical studies have only analyzed the KO to K step, as the second reaction is difficult to study because of the rapid oxidation of KH2. A cellular approach has also been used; however, almost all studies have been performed in cells containing the warfarin-resistant quinone reductase, which confounds analyzing VKORC1-specific K to KH2 reduction.12-14 The catalytic mechanisms of KO to K and K to KH2 are not the same,15 and could potentially be differently affected by warfarin. Bacterial orthologs of VKORC1 require millimolar warfarin to inhibit the K to KH2 reaction,16 which contrasts nanomolar amounts that inhibit mammalian VKORC1 KO to K reduction and raises the question of warfarin sensitivity for the K to KH2 reaction. These considerations highlighted the need for definitive studies to gain an understanding of warfarin inhibition relevant to what actually occurs during anticoagulant therapy.
The unknown mechanism for warfarin inhibition is of interest regarding the properties of a VKORC1 mutant, Y139F, isolated from warfarin-resistant rats. Y139F required much higher doses of warfarin than wild-type (wt) enzyme to inhibit KO to K reduction when assayed biochemically.17 However, Y139F did not support VKD protein carboxylation in cells containing warfarin.18 This insufficiency led to the conclusion that the normal role for wt VKORC1 is primarily KO to K reduction, and that an enzyme other than VKORC1 is responsible for the K to KH2 step.18 The subsequent demonstration that wt VKORC1 efficiently performs both reactions6 indicated that a different explanation was likely. We therefore investigated Y139F, as well as wt VKORC1 and the warfarin-resistant human mutant Y139H. The studies led to the discovery that warfarin uncouples normal reduction (ie, inhibiting full KO to KH2 reduction much more than expected from the inhibition of the 2 individual reactions). Our results suggest that during warfarin therapy, full KO reduction shifts from a mechanism that requires only VKORC1 to a mechanism that also involves a second reductase. This activity may not be ubiquitously expressed similar to VKORC1,19 and tissues may therefore respond differently to warfarin in their efficiency of VKD protein carboxylation.
Methods
Generation and characterization of mammalian cell lines expressing wt or mutant VKORC1
BHK cells expressing r-factor IX (fIX)20 were transfected with r-wt VKORC1/ZEM229 and selected using methotrexate (0.15-1 µM; Calbiochem). Clones were screened for expression using anti-VKORC1 antibody, and positives were then assayed for activity by measuring the carboxylation of secreted fIX or intracellular KO to K reduction. Cells (107) were incubated in 5 mL KO (2 µM)-containing serum free media for 6 hours and then exchanged into fresh media to remove residual bovine fIX. After 18 hours, media (1 mL) were concentrated (Millipore) and then digested with PNGase F (New England Biolabs) to separate fIX from a background band. Western analysis was performed using fIX standards (Enzyme Research Laboratories) and antibodies against Gla (5 µg/mL; BioMedica Diagnostics) or fIX (0.4 µg/mL).20,21 Quantitation was performed using an Odyssey LiCor scanner. To monitor KO to K reduction, trypsinized cells were rinsed twice in cold PBS (10 mL), centrifuged (2000g, 5 minutes), and resuspended in 500 µL cold 25 mM sodium phosphate at pH 7.9, 25 mM KCl, 20% glycerol, 0.75% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate) (Sigma), and 1 mM PMSF. K25 (1.6 nmol, GL Synthesis) was added, the samples were centrifuged (3500g, 15 minutes), and vitamin K was then extracted and analyzed by high-performance liquid chromatography in parallel with standards (KO, K, K25), as before.22
A r-wt VKORC1/r-fIX BHK cell line that gave a twofold increase in both fIX carboxylation and KO to K reduction was subsequently used to screen r-Y139F/r-fIX BHK and r-Y139H/r-fIX BHK clones. Cells were generated as earlier and screened by western analysis with anti-VKORC1 antibody to select clones with the same level of expression as the r-wt VKORC1.
Warfarin inhibition of VKORC1 activity in mammalian cells
Cells were incubated in serum-free media (5 mL), KO (2 µM), and warfarin (1.5-2000 nM; the range varied for the individual cell lines). Duplicate plates were analyzed for each warfarin concentration, and cells lacking vitamin K were included as a control. Cells were incubated for 6 hours and then exchanged into fresh media and incubated for an additional 18 hours. Intracellular KO to K reduction and carboxylation of secreted fIX were then analyzed as described earlier. Media were also monitored in a clotting assay to determine the activity of the secreted fIX, as previously described.23
The effect of warfarin on K reduction was tested at the decreased K concentration resulting from inhibition of KO reduction. Cells were incubated with or without warfarin (50 nM for r-wt VKORC1/r-fIX BHK cells or 1 µM for r-Y139F/r-fIX BHK cells) and KO (2 µM) or K (0.4-2 µM). The intracellular K concentration and fIX carboxylation were then determined as described earlier. Cells that were incubated in K without warfarin and that contained the same intracellular K concentration as those incubated with KO plus warfarin were then compared for warfarin inhibition of fIX carboxylation.
Results
The Y139F VKORC1 mutant is functional for full reduction of KO to KH2
The Y139F mutant was not warfarin resistant for supporting carboxylation in cells,18 and we assessed whether human VKORC1 bearing Y139F is dysfunctional for normal reduction of KO to KH2. We used previously developed methods that assess full reduction of KO to KH2 by performing the entire analysis under nitrogen.6 Purified Y139F was incubated with KO and reductant, and vitamin K forms were then isolated and analyzed by high-performance liquid chromatography. The reductant was a thioredoxin-based system that substitutes for the unknown, natural redox protein that activates VKORC1.22 Purified Y139F converted KO to both K and KH2 (Figure 1B).
Y139F was also tested for the ability to support carboxylation, which depends on KH2 production. Microsomes were prepared from insect cells, which lack endogenous carboxylation components, coinfected with baculoviruses containing the carboxylase and either wt or Y139F VKORC1. The microsomes were incubated with KO, the thioredoxin system, and a carboxylase peptide substrate (FLEEL), and carboxylation was assessed by monitoring [14C]-CO2 incorporation into FLEEL. Microsomes were used because VKORC1-carboxylase interaction requires intact membranes.6 Y139F supported carboxylation (Figure 1C), which was not observed with control microsomes containing only the carboxylase. The Y139F-specific activity was 35% that of wt VKORC1, whereas 100% activity was observed with KH2 that bypasses VKORC1 and is used directly by the carboxylase (Figure 1C-D). To assess whether insect cells contain a quinone reductase that could account for K to KH2 reduction, the control microsomes containing only the carboxylase were also incubated with K. Carboxylation was not observed (Figure 1C), showing that Y139F drives this reaction. Similar results were obtained with an assay measuring KO reduction (supplemental Figure 1, available on the Blood Web site). Immunoprecipitation analysis indicated a Y139F dimer (Figure 1E), which in wt VKORC1 facilitates full KO reduction.6
Warfarin inhibits carboxylation that depends on full VKORC1 reduction much more than expected from the inhibition of 2 reactions
The Y139F mutant supported carboxylation in the absence of warfarin (Figure 1), but not in cells containing warfarin,18 and we therefore assessed the effect of warfarin on Y139F activity. When assayed for the reduction of KO to KH2, the warfarin-resistant Y139F mutant was strongly inhibited by warfarin (Figure 2A). Carboxylation was also studied, which allowed a much larger number of samples to be analyzed. VKORC1 is rate limiting for carboxylation in the assay,6 and carboxylation therefore reflects VKORC1 activity. Microsomes containing Y139F and the carboxylase were incubated with KO, the thioredoxin system, and warfarin, and carboxylation was monitored by measuring [14C]-CO2 incorporation into FLEEL. KO reduction to K was also monitored, and was performed under nitrogen to prevent carboxylase recycling of KH2 to KO. Both assays were performed under steady-state conditions (supplemental Figure 2). A comparison of the 2 Y139F reactions showed that carboxylation was abolished at warfarin concentrations at which Y139F retained significant levels of KO to K activity (Figure 2B).
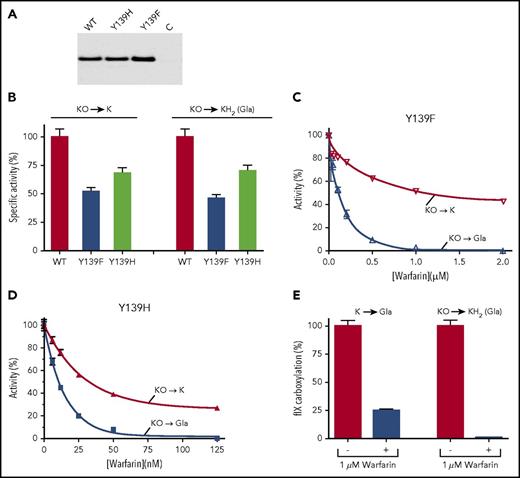
Warfarin inhibits full VKORC1 reduction and consequent carboxylation much more than expected from the inhibition of the 2 individual reactions. (A) Y139F was assayed under nitrogen for KO to KH2 reduction in the absence (−) or presence (+) of warfarin. (B-D) Microsomes containing the carboxylase and Y139F or Y139H or wt VKORC1 were assayed for KO reduction to K, using high-performance liquid chromatography to monitor vitamin K forms, and for carboxylation by measuring [14C]-CO2 incorporation into FLEEL. (E-F) The K to KH2 reaction was analyzed by first assaying microsomes containing the carboxylase and wt VKORC1 (E) or Y139F (F) for KO to K inhibition, which gave the indicated concentrations of K. Carboxylation that depends on K to KH2 reduction was then monitored for warfarin inhibition with these K concentrations, or with KO (65 µM).
Warfarin inhibits full VKORC1 reduction and consequent carboxylation much more than expected from the inhibition of the 2 individual reactions. (A) Y139F was assayed under nitrogen for KO to KH2 reduction in the absence (−) or presence (+) of warfarin. (B-D) Microsomes containing the carboxylase and Y139F or Y139H or wt VKORC1 were assayed for KO reduction to K, using high-performance liquid chromatography to monitor vitamin K forms, and for carboxylation by measuring [14C]-CO2 incorporation into FLEEL. (E-F) The K to KH2 reaction was analyzed by first assaying microsomes containing the carboxylase and wt VKORC1 (E) or Y139F (F) for KO to K inhibition, which gave the indicated concentrations of K. Carboxylation that depends on K to KH2 reduction was then monitored for warfarin inhibition with these K concentrations, or with KO (65 µM).
Substitution of Tyr139 by a different residue, His, causes warfarin resistance in humans,24 and we also tested the Y139H mutant. Y139H had a specific activity similar to Y139F (supplemental Figure 3) and showed the same differential response for full vs partial KO reduction. Gla production was eliminated at a warfarin concentration (1 µM), where KO to K reduction was substantial (40%; Figure 2C). Wild-type VKORC1 was also tested and showed similar results (Figure 2D), indicating that the differential response of carboxylation and KO reduction to warfarin is a general property of VKORC1.
Loss of carboxylation could be not only a result of the inhibition of KO to K but also the consequent decrease in K concentration and to the inhibition of the K to KH2 reaction. We therefore tested warfarin inhibition of K to KH2 (ie, Gla production) at the low K concentration resulting from inhibition of the KO to K reaction. The K concentration was determined by incubating microsomes containing VKORC1 and the carboxylase with KO (65 µM) in the presence or absence of warfarin (1 µM). Warfarin resulted in a K concentration of 0.2 µM. When warfarin (1 µM) inhibition was tested using this K concentration, carboxylation was decreased threefold (Figure 2E). This value was much less than the 50-fold decrease in carboxylation observed with KO (65 µM) substrate. Similar results were obtained with the Y139F mutant (Figure 2F). The decrease in carboxylation mediated by full VKORC1 reduction was therefore much more than expected from the inhibition of the 2 reactions, indicating that warfarin uncouples the reactions.
The effect of warfarin on full VKORC1 reduction vs the individual reactions in cells indicates uncoupling of the 2 reactions
Cellular analysis required a cell line that does not contain a warfarin-resistant quinone reductase, which would interfere with monitoring the effect of warfarin on VKORC1-mediated K to KH2 reduction. BHK cells lack this activity, as shown by analysis of the VKD protein fIX. Secreted protein was monitored for fIX carboxylation, using an antibody against Gla,21 and for total fIX, using an antibody that recognizes both carboxylated and uncarboxylated fIX.20 Warfarin eliminated fIX carboxylation in cells containing K and warfarin, and in control cells containing KO that requires VKORC1 (Figure 3A), indicating that a warfarin-resistant quinone reductase was not present. Consistent with the result, we found that the VKORC1L1 paralog reported to be warfarin resistant25 was not expressed (data not shown). We note that fIX was used to study carboxylation because fIX is secreted regardless of the extent of carboxylation,20 and therefore secreted fIX carboxylation accurately represents the product of intracellular carboxylation.
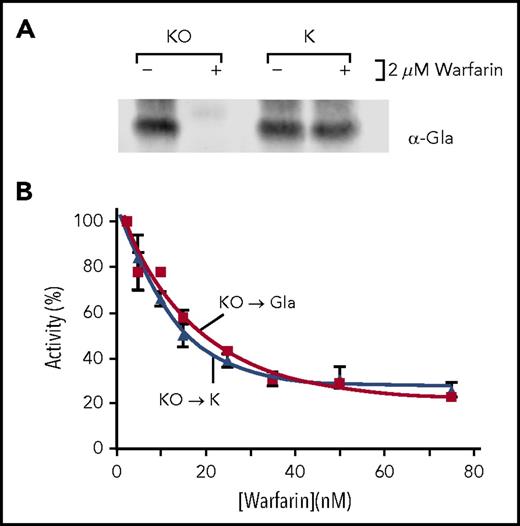
Warfarin uncouples the 2 VKORC1 reactions that fully reduce KO to KH2. (A) r-wt VKORC1/r-fIX BHK cells were incubated without vitamin K (−K) or with KO or K (both at 2 µM) in the absence (−) or presence (+) of warfarin (2 µM). Secreted fIX was analyzed in westerns for carboxylation (anti-Gla21 ) or total fIX (anti-fIX). The differences in fIX migration are a result of glycosylation.34 (B) r-fIX BHK and r-wt VKORC1/r-fIX BHK cells incubated with KO (2 µM) were monitored for KO reduction by isolation of intracellular vitamin K, followed by high-performance liquid chromatography analysis to separate and quantitate individual forms. fIX carboxylation was monitored by western analysis with anti-Gla antibody. (C) r-wt VKORC1/r-fIX BHK cells were incubated with KO (2 µM) and varying amounts of warfarin. KO reduction and fIX carboxylation were monitored as in (B). The KO to K curve is only slightly affected by subsequent K reduction, as only ∼15% of the K resulted in Gla product. (D) The K to KH2 reaction and consequent carboxylation was analyzed using low K levels that result from inhibition of the KO to K reaction. Cells were incubated with warfarin (50 nM) and KO (2 µM) or K (0.4-2 µM). A range of K concentrations was used to identify cells with the appropriate intracellular K concentration for comparison with cells incubated in KO. Warfarin decreased KO to K reduction 2.5-fold, giving an intracellular K level of 1 nmol for 107 cells. Left: warfarin (50 nM) inhibition of carboxylation in cells containing this intracellular K level. Right: warfarin inhibition of cells incubated with KO (2 µM).
Warfarin uncouples the 2 VKORC1 reactions that fully reduce KO to KH2. (A) r-wt VKORC1/r-fIX BHK cells were incubated without vitamin K (−K) or with KO or K (both at 2 µM) in the absence (−) or presence (+) of warfarin (2 µM). Secreted fIX was analyzed in westerns for carboxylation (anti-Gla21 ) or total fIX (anti-fIX). The differences in fIX migration are a result of glycosylation.34 (B) r-fIX BHK and r-wt VKORC1/r-fIX BHK cells incubated with KO (2 µM) were monitored for KO reduction by isolation of intracellular vitamin K, followed by high-performance liquid chromatography analysis to separate and quantitate individual forms. fIX carboxylation was monitored by western analysis with anti-Gla antibody. (C) r-wt VKORC1/r-fIX BHK cells were incubated with KO (2 µM) and varying amounts of warfarin. KO reduction and fIX carboxylation were monitored as in (B). The KO to K curve is only slightly affected by subsequent K reduction, as only ∼15% of the K resulted in Gla product. (D) The K to KH2 reaction and consequent carboxylation was analyzed using low K levels that result from inhibition of the KO to K reaction. Cells were incubated with warfarin (50 nM) and KO (2 µM) or K (0.4-2 µM). A range of K concentrations was used to identify cells with the appropriate intracellular K concentration for comparison with cells incubated in KO. Warfarin decreased KO to K reduction 2.5-fold, giving an intracellular K level of 1 nmol for 107 cells. Left: warfarin (50 nM) inhibition of carboxylation in cells containing this intracellular K level. Right: warfarin inhibition of cells incubated with KO (2 µM).
The r-fIX BHK cells were transfected with r-human VKORC1, which was untagged because epitope tags resulted in very low expression for unknown reasons. Transfected cells were first screened using an antibody that recognizes human but not endogenous hamster VKORC1, and were then assayed for intracellular KO to K reduction. The goal was to identify clones expressing similar levels of recombinant and endogenous VKORC1, with overall expression being sufficiently low so that VKORC1 is the rate-limiting step in carboxylation, as before.26 An appropriate clone was identified that gave twofold higher levels of both KO reduction and fIX carboxylation in r-wt VKORC1/r-fIX BHK cells than in the progenitor r-fIX BHK cells (Figure 3B).
The effect of warfarin was then tested by incubating r-wt VKORC1/r-fIX cells in media containing KO and warfarin, and then monitoring carboxylation and KO reduction. Warfarin decreased the carboxylation of secreted fIX without affecting the levels of fIX (supplemental Figure 4). At the highest warfarin concentration tested (50 nM), carboxylation was undetectable (Figure 3C), which represented at least a 200-fold decrease in carboxylation. In contrast, KO reduction was only reduced threefold (Figure 3C). Carboxylation that requires full VKORC1 reduction was therefore much more sensitive to warfarin than KO reduction.
The effect of warfarin on the K to KH2 step was also tested, using the decreased K concentration that results from inhibition of the KO to K reaction. r-wt VKORC1/r-fIX cells were incubated with KO (2 µM) and a concentration of warfarin (50 nM) that eliminated carboxylation while retaining KO to K activity (Figure 3C). Warfarin inhibited K production threefold to give an intracellular level of 1 nmol K for the 107 cells studied. In cells containing this level of K and treated with warfarin (50 nM), fIX carboxylation was inhibited 2.5-fold (Figure 3D). In contrast, fIX carboxylation was undetectable in cells incubated with KO (2 µM), representing a 70-fold decrease in activity. Warfarin inhibition of full KO to KH2 reduction is therefore surprisingly higher than expected from the inhibition of 2 reactions, implicating uncoupling of the reactions by warfarin.
r-Y139F/r-fIX and r-Y139H/r-fIX BHK cells were also tested for warfarin sensitivity. The mutant VKORC1s were expressed at similar levels to that of r-wt VKORC1 (Figure 4A), and had specific activities 50% to 70% that of wt VKORC1 (Figure 4B). Both cell lines showed similar responses as r-wt VKORC1/r-fIX BHK cells to warfarin (Figure 4C-D). r-Y139F/r-fIX BHK cells, for example, showed a twofold decrease in KO reduction at a warfarin concentration (1 µM) that eliminated carboxylation, which represented at least a 400-fold decrease. The r-Y139F/r-fIX BHK cells were further analyzed for K to KH2 reduction by first determining the decrease in K levels resulting from inhibition of the first reaction, and then testing warfarin inhibition of K-mediated carboxylation at the decreased concentration. Carboxylation that required full KO to KH2 reduction was much more inhibited than that requiring only the K to KH2 reaction (Figure 4E).
Warfarin uncouples full KO to KH2reduction in cells expressing warfarin-resistant mutants. (A) Western analysis with anti-VKORC1 antibody was performed on r-fIX BHK cells expressing r-VKORC1 variants or untransfected cells (C). (B) Specific activities were determined by assaying the cells as in Figure 3B and normalizing activity to protein expression. (C-D) Warfarin sensitivity was monitored as in Figure 3C. (E) Warfarin inhibition of carboxylation dependent on the Y139F K to KH2 reaction was tested as in Figure 3D.
Warfarin uncouples full KO to KH2reduction in cells expressing warfarin-resistant mutants. (A) Western analysis with anti-VKORC1 antibody was performed on r-fIX BHK cells expressing r-VKORC1 variants or untransfected cells (C). (B) Specific activities were determined by assaying the cells as in Figure 3B and normalizing activity to protein expression. (C-D) Warfarin sensitivity was monitored as in Figure 3C. (E) Warfarin inhibition of carboxylation dependent on the Y139F K to KH2 reaction was tested as in Figure 3D.
Y139H becomes more active than wt VKORC1 in the presence of warfarin
The Y139H mutant had impaired activity (Figure 4B; supplemental Figure 3), but would be expected to be more active than wt VKORC1 to explain warfarin resistance in patients. We tested whether the relative activities change in the presence of warfarin. A dose-response experiment was performed for r-Y139H/r-fIX BHK cells using a range of warfarin concentrations more similar to that of r-wt VKORC1 BHK cells. Y139H was more warfarin resistant than wt VKORC1, which was more pronounced at higher warfarin concentrations (Figure 5A). This observation may reflect VKORC1 populations that will respond differently as warfarin levels increase: r-wt VKOR/r-fIX cells express only wt VKORC1, whereas r-Y139H/r-fIX cells contain a mixture of forms that are sensitive or resistant to warfarin. A comparison of the activities showed that Y139H became more active than wt VKORC1 at the higher warfarin concentrations (Figure 5B). The clotting activity of fIX was also monitored and gave similar results (Figure 5C). Higher activity in the presence of warfarin can therefore explain warfarin resistance for the Y139H mutant.
Y139H is only more active than wt VKORC1 in the presence of warfarin. (A) The response of VKORC1-dependent fIX carboxylation to warfarin was monitored for wt and Y139H VKORC1. (B) The relative activities were determined by normalizing the fIX carboxylation values to protein levels determined by western analysis with anti-VKORC1 antibody. (C) The same samples were tested in a clotting assay.
Y139H is only more active than wt VKORC1 in the presence of warfarin. (A) The response of VKORC1-dependent fIX carboxylation to warfarin was monitored for wt and Y139H VKORC1. (B) The relative activities were determined by normalizing the fIX carboxylation values to protein levels determined by western analysis with anti-VKORC1 antibody. (C) The same samples were tested in a clotting assay.
293 cells expressing warfarin-resistant quinone reductase activity show similar warfarin inhibition of fIX carboxylation and KO reduction
The BHK cells in which warfarin uncoupling was revealed lacked warfarin-resistant quinone reductase activity (Figure 3A), and we tested a different line, 293 cells, that contains this activity. Activity was shown by culturing r-fIX 293 cells in warfarin and K (Figure 6A). These cells were also tested for expression of VKORC1L1, which was not detected (supplemental Figure 5), indicating that virtually all KO reduction in the cells is a result of VKORC1. r-FIX 293 cells were next tested for KO reduction and Gla production in cells containing KO and varying concentrations of warfarin. The results were strikingly different from those obtained in the BHK cells: warfarin inhibited both activities to the same extent, and significant levels of fIX carboxylation were observed at high warfarin concentrations (Figure 6B). Disruption of fIX carboxylation that requires full KO to KH2 reduction in the absence of the warfarin-resistant quinone reductase (Figures 2-4), but not its presence (Figure 6), strongly suggests that this activity facilitates full reduction by performing the K to KH2 reaction.
293 cells that have warfarin-resistant quinone reductase activity do not show differences in inhibition of KO reduction and fIX carboxylation. (A) r-FIX 293 cells were generated by transfection of human r-fIX in pCMV6-A-Puro (Origene) and selection with puromycin (1 µg/mL). A clonal isolate was tested for warfarin inhibition as in Figure 3A, revealing the presence of warfarin-resistant quinone reductase activity. (B) Warfarin sensitivity of KO reduction and fIX carboxylation were analyzed as in Figure 3C.
293 cells that have warfarin-resistant quinone reductase activity do not show differences in inhibition of KO reduction and fIX carboxylation. (A) r-FIX 293 cells were generated by transfection of human r-fIX in pCMV6-A-Puro (Origene) and selection with puromycin (1 µg/mL). A clonal isolate was tested for warfarin inhibition as in Figure 3A, revealing the presence of warfarin-resistant quinone reductase activity. (B) Warfarin sensitivity of KO reduction and fIX carboxylation were analyzed as in Figure 3C.
Discussion
Warfarin suppresses VKORC1 function in millions of people by a mechanism that is poorly understood, and this work reveals a surprising property of warfarin inhibition. VKORC1 performs 2 reactions to fully reduce KO to the KH2 cofactor required for carboxylation, and in what are the first studies to analyze full reduction vs the individual reactions, we found that warfarin uncouples these reactions. Carboxylation that requires full reduction was eliminated at warfarin concentrations at which significant levels of KO to K reduction were observed (Figures 2B-D, 3C, and 4C-D). The K to KH2 reaction was also inhibited much less than full reduction (Figures 2E-F, 3D, and 4E) when tested with low K concentrations that result from inhibition of the KO to K reaction. Warfarin therefore inhibits full reduction much more than expected from the combined inhibition of 2 reactions, implicating an uncoupling mechanism. This work explains the previous enigmatic observation that the warfarin-resistant Y139F mutant did not support VKD protein carboxylation in cells containing warfarin18 : the mutant fully reduced KO to KH2 in the absence of warfarin (Figure 1B), but was disrupted when warfarin was present (Figures 2 and 4). Our discovery of an unexpected facet of warfarin inhibition has important implications for patients on warfarin therapy and also raises several new questions about the mechanism of warfarin inhibition.
Uncoupling was revealed by the absence of a second activity in the biochemical and cellular analyses, a warfarin-resistant quinone reductase, and different results were obtained when this activity was present. r-wt VKORC1/r-fIX BHK and r-fIX 293 cells showed similar levels of warfarin inhibition of KO reduction to K; however, fIX carboxylation was abolished in r-wt VKORC1/r-fIX BHK, but not in r-fIX 293 cells (Figures 3 and 6). The difference in results can be explained by the warfarin-resistant quinone reductase in the r-fIX 293 cells performing the K to KH2 reaction to facilitate carboxylation.
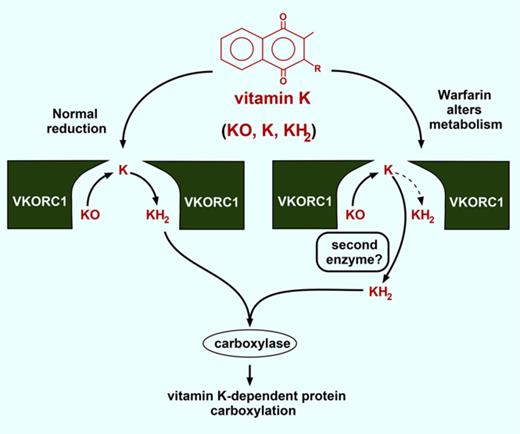
The results suggest that vitamin K metabolism changes during warfarin therapy. Normally, VKORC1 fully reduces KO to KH2 (Figure 7A). The efficiency is high, as KO-supported carboxylation approached the level observed with saturating amounts of KH2 (Figure 1C-D). Sequestration of the K intermediate in the VKORC1 dimer could contribute to efficiency by concentrating K for reduction to KH2. Warfarin uncouples the normal process of KO to KH2 reduction, and future studies will be of interest to determine the mechanism of uncoupling. One possibility is warfarin interfering with K movement between the 2 subunits. Alternatively, warfarin may impair K and KO binding differently.
How is full reduction of KO to KH2accomplished during warfarin therapy? (A) VKORC1 normally performs 2 reactions to generate the KH2 cofactor required for VKD protein carboxylation. (B) During warfarin therapy, a warfarin-resistant quinone reductase may cooperate with VKORC1 to produce KH2.
How is full reduction of KO to KH2accomplished during warfarin therapy? (A) VKORC1 normally performs 2 reactions to generate the KH2 cofactor required for VKD protein carboxylation. (B) During warfarin therapy, a warfarin-resistant quinone reductase may cooperate with VKORC1 to produce KH2.
The results raise the question of whether a second reductase is required to accomplish full reduction during warfarin therapy. The Y139H that causes warfarin resistance in humans only showed resistance at high warfarin concentrations (Figure 5) where uncoupling was extensive (Figure 4D), suggesting the need for a second enzyme. The warfarin-resistant quinone reductase is an obvious candidate for the second enzyme. This reductase was identified by the administration of large amounts of vitamin K to patients, and is thought to support carboxylation independent of VKORC1.4,27,28 During normal therapy, this enzyme alone would not be effective because dietary vitamin K levels are low and would be rapidly converted to KO by the carboxylase. However, the quinone reductase could be functionally significant in cooperation with VKORC1 that recycles KO. A key observation in these studies is that warfarin-inhibited VKORC1 retains substantially more KO to K activity than previously appreciated. Warfarin therapy monitors clotting, and even when clotting was low (Figure 5C), there were significant levels of the K intermediate (Figures 3 and 4). Importantly, VKORC1 generates the K intermediate precisely where it is needed; that is, in the endoplasmic reticulum membrane where carboxylation occurs. As a consequence, even though dietary levels of K are low, the local levels are high and continuously supplied by VKORC1 recycling of KO. An intriguing possibility is that VKORC1 drives the activity of the quinone reductase to support carboxylation even during normal warfarin therapy, when dietary vitamin K levels are low (Figure 7B).
A need for 2 enzymes to accomplish full KO reduction would have important implications for extrahepatic VKD protein function during warfarin therapy. Only the hemostatic VKD proteins secreted from liver are monitored; however, virtually all tissues express VKD proteins.1 Normally, a single enzyme can support carboxylation throughout the body because VKORC1 is ubiquitously expressed19 and able to fully reduce KO to KH2.6 During warfarin therapy, all tissues would show a similar decrease in carboxylation if VKORC1 alone is responsible for generating KH2. However, there would be differential effects if 2 enzymes are required because VKD protein carboxylation would be negligible in those tissues lacking the second enzyme. This scenario could explain the calcification defects observed during warfarin therapy.29,30 Dysfunction is thought to be at least in part a result of undercarboxylation of matrix Gla protein, a VKD protein that regulates calcification.31
The observation that Y139H only became more active than wt VKORC1 when warfarin was present (Figure 5) provides a novel explanation for warfarin resistance. Why VKORC1 mutations cause warfarin resistance has been puzzling, because mutations typically impair activity, whereas resistance implies higher activity; our data can explain this apparent dichotomy. Future studies will be of interest as to whether this mechanism applies to other mutants. Some mutants have been proposed to be more active than wt VKORC112,14 ; however, there is disagreement on the activities. For example, 10 mutants were found to be inactive in a direct biochemical assay that measured KO to K reduction,32 but were fully active in cellular studies.14 The cell assay is indirect; that is, studying VKD protein carboxylation that will only be proportional to VKORC1 activity if VKORC1 performs the rate-limiting step. VKORC1 is rate limiting with low level overexpression (eg, Figure 3B), whereas higher overexpression results in the carboxylase becoming rate limiting.26 The level of r-VKORC1 overexpression was not monitored in the cell studies,12-14,33 making the mutant activities difficult to interpret. Defining these activities will be significant for understanding both VKORC1 function and warfarin resistance.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants R01HL55566 and R01HL81093 (K.L.B.). K.W.R. was supported by National Institutes of Health, National Institute on Aging grant R01AG051601 and National Science Foundation grant 1516220.
Authorship
Contribution: M.A.R. and K.L.B. designed the experiments, with input from K.W.R.; M.A.R., K.W.H., L.W., and S.S. performed the experiments; and K.L.B. wrote the manuscript, with input from M.A.R. and K.W.R.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kathleen L. Berkner, Cleveland Clinic Foundation, Lerner Research Institute, Department of Molecular Cardiology, 9500 Euclid Ave, NB50, Cleveland, OH 44195; e-mail: berknek@ccf.org.

![Figure 1. The Y139F VKORC1 mutant fully reduces KO to KH2 to drive carboxylation. (A) Cycling between oxidized (KO) and reduced (KH2) vitamin K drives VKD protein carboxylation by the γ-glutamyl carboxylase. VKORC1 fully reduces KO to the quinone intermediate (K) and then to KH2; an unknown warfarin-resistant quinone reductase can also perform the second reaction. (B) Purified human VKORC1 bearing the Y139F mutation was tested for KO reduction to KH2 by performing all steps under nitrogen to block KH2 oxidation. (C) Microsomes containing only the carboxylase (Carb) or also expressing wt or Y139F VKORC1 were tested for KO or K supported carboxylation, as assessed by [14C]-CO2 incorporation into the peptide FLEEL. (D) The microsomes in panel C were also tested for KH2-supported carboxylation. (E) Insect cells containing tagged (Y139Fflag) and untagged Y139F or only the individual forms were subjected to immunopurification with anti-FLAG antibody, followed by western analysis using antibody against VKORC1, as before.22 Mock indicates uninfected cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/25/10.1182_blood-2017-09-804666/4/m_blood804666f1.jpeg?Expires=1767748587&Signature=YsEjsOFECV~KucsbkL2t27DgLgClHjwqvTyLLYnZ1KfQgPIRuiPjhezlFQr~neRqSG5uy1dSnXSswHayj5l0QCJITnjq-KSwvS-5xeZi13Rk5r3cj1IUeCg2ZeM8qXCljmlu7FyNPU6y0oPdyLMABNPrjVAcVPu7edpra3h~5FeVgoaoooRfNjt6ehZ4lygFQidr4lPjh4rUeJmonOmzyADXNere3nZ8QV1emFhF3MLZN8PoFUE74wlYlZgj2uv-jUcEQWbRB4s~9diqsY74PyfoXZRmfPo-3ZTgYzz9BX39w9BQg8tVecMjDdznTN589Au4WjKaaqfMxldzpA8~~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Warfarin inhibits full VKORC1 reduction and consequent carboxylation much more than expected from the inhibition of the 2 individual reactions. (A) Y139F was assayed under nitrogen for KO to KH2 reduction in the absence (−) or presence (+) of warfarin. (B-D) Microsomes containing the carboxylase and Y139F or Y139H or wt VKORC1 were assayed for KO reduction to K, using high-performance liquid chromatography to monitor vitamin K forms, and for carboxylation by measuring [14C]-CO2 incorporation into FLEEL. (E-F) The K to KH2 reaction was analyzed by first assaying microsomes containing the carboxylase and wt VKORC1 (E) or Y139F (F) for KO to K inhibition, which gave the indicated concentrations of K. Carboxylation that depends on K to KH2 reduction was then monitored for warfarin inhibition with these K concentrations, or with KO (65 µM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/25/10.1182_blood-2017-09-804666/4/m_blood804666f2.jpeg?Expires=1767748587&Signature=ifdq8g3xbqAHGS5muQwcpfBr1KwhB993fa8W~ZM~tQcGRS6mZQAlOTHG4U3UmReMkiQyeodQcNivPn1WIsTvYloYuJIpfNQvEq51ObK5PbyjT60Ke29sfc4QjfPdtscAGb0nTaawVddeNOJa2ATWobZjOldXX20oTP~f~X7N0EqLTBuqQiR5l~8G0L96P1bXan4~CW~bN80Nk~Dd1WcfbVI1MiPgf8STOhygbVPYRsFyqRxO-cn9jeAEkDS91F~iPvXkv32Qix06S5srhX7~LPJJ-weOLnR5HK5mzxdrMKCvNGYxMfklLKrxkiwixoHRkwect8kkkCUTKit-zq52hQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)