Key Points
NRF2 knockout inhibits fetal hemoglobin expression during gestational erythropoiesis in SCD mice.
Loss of the cellular antioxidant response mediated by NRF2 exacerbates spleen damage, inflammation, and oxidative stress in SCD mice.
Abstract
The basic leucine zipper transcription factor nuclear factor (erythroid-derived 2)-like 2 (NRF2) plays a critical role in the cellular antioxidant response under oxidative stress conditions. In this study, we investigated the role of NRF2 in fetal hemoglobin expression and the pathophysiology of sickle cell disease (SCD) in a NRF2 knockout (SCD/NRF2−/−) transgenic mouse model. NRF2 loss impaired survival of SCD pups during gestation and in the first 2 months of life. Furthermore, fetal hemoglobin expression was inhibited during erythropoiesis in embryonic day 13.5 and embryonic day 18.5 fetal liver and adult spleen and bone marrow cells, respectively. Examination of peripheral red blood cells revealed an increase of reactive oxygen species (ROS) and sickling under hypoxic conditions. Loss of NRF2 function in SCD/NRF2−/− mice produced greater splenomegaly with red pulp expansion and obscured architecture. In addition, NRF2 knockout reduced the expression of its target antioxidant proteins, leading to increased levels of ROS, proinflammatory cytokines, and adhesion molecules in SCD mice. Genetic knockout of NRF2 demonstrates its role in developmentally regulated γ-globin gene expression and the ability to control oxidative stress and the phenotypic severity of SCD.
Introduction
Sickle cell disease (SCD) is a group of inherited blood disorders with a wide range of clinical symptoms caused by polymerization of hemoglobin S (HbS) in red blood cells (RBCs) and chronic hemolysis leading to anemia, inflammation, and elevated reactive oxygen species (ROS).1,2 Previously, nuclear factor (erythroid-derived 2)-like 2 (NRF2), the master regulator of the cellular oxidative stress response,3 was shown to mediate γ-globin gene activation4-6 through direct promoter interactions.6 More recently, we demonstrated the ability of NRF2 to bind the γ-globin promoter and locus control region to mediate chromatin looping during normal erythropoiesis.7 These findings were complemented by in vivo genetic8 and pharmacologic9-12 activation of NRF2, which reduces intravascular hemolysis to improve the phenotype of SCD mice.
To expand on these findings, we investigated the effects of NRF2 loss on fetal hemoglobin (HbF) expression during gestational erythropoiesis and severity of the SCD phenotype using a transgenic mouse model. Our data demonstrated that genetic NRF2 knockout silenced γ-globin gene transcription and HbF expression during development while exacerbating oxidative stress and proinflammatory phenotypes in adult SCD mice. These findings confirm the critical role of NRF2 in hemoglobin regulation and warrant the development of therapeutic agents that activate NRF2 expression as a strategy for SCD treatment.
Study design
Animals
The humanized Townes SCD mouse model (B6;129-Hbatm1(HBA)TowHbbtm2(HBG1,HBB*)Tow/Hbbtm3(HBG1,HBB)Tow/J) was characterized previously.13 The C57BL/6J NRF2 knockout (NRF2−/−) mouse14 was provided by Dr. Yamamoto (Tohoku University, Sendai, Japan). The SCD and NRF2−/− mice were crossbred to generate the SCD/NRF2−/− mouse (supplemental Methods; supplemental Figure 1A; available on the Blood Web site) after approval by the Augusta University Institutional Animal Care and Use Committee.
See supplemental Methods for additional details.
Results and discussion
To assess the effect of NRF2 function on the pathophysiology of SCD, we established the SCD/NRF2−/− mouse model (supplemental Figure 1). Although the NRF2 gene is transmitted through Mendelian genetics, NRF2−/− produced variable penetrance demonstrated by 2% SCD/NRF2−/− pups (supplemental Figure 1B), supporting embryonic lethality on a SCD background. In addition, the fertility of female SCD/NRF2−/− mice was decreased, with smaller litter sizes and high mortality in the perinatal period (supplemental Figure 1C-D). These data support a role for NRF2 in fetal and perinatal survival for SCD mice.
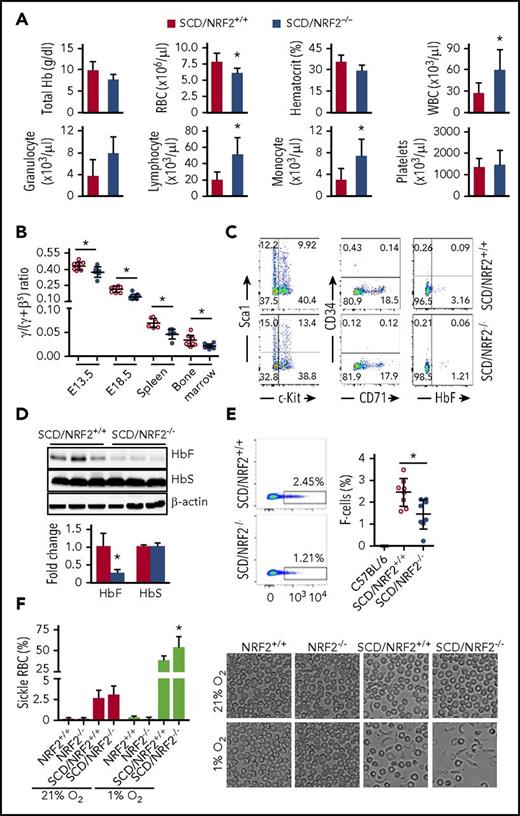
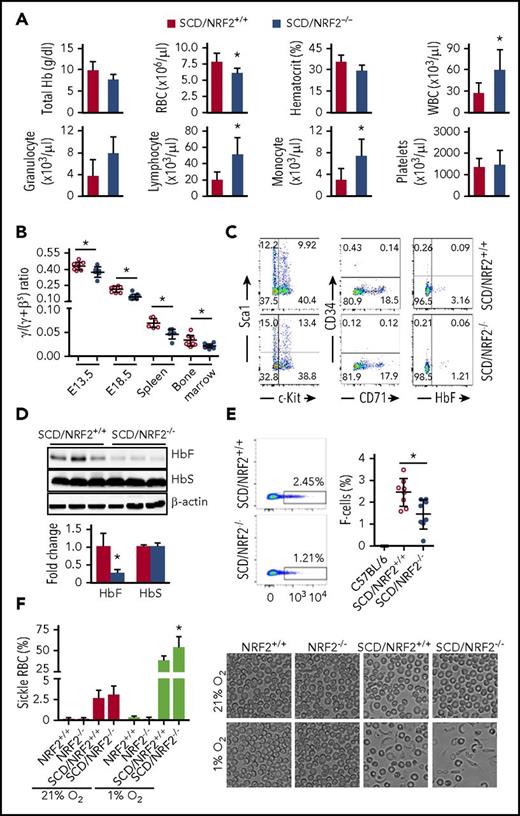
To evaluate the hematopoietic effect, we performed complete blood cell counts with differentials, which confirmed a significant decrease in RBCs and an increase in total white blood cells along with lymphocytes and monocytes in SCD/NRF2−/− mice compared with SCD/NRF2+/+ mice (Figure 1A); other hematologic parameters were not affected. We next examined globin gene expression during development. The γ-globin messenger RNA (mRNA) levels were significantly lower in comparison with βS-globin mRNA (Figure 1B), and the γ:(γ+βS) globin ratios in SCD/NRF2−/− mice was decreased in embryonic day 13.5 and embryonic day 18.5 fetal liver cells from 0.44% to 0.37% and from 0.21% to 0.14% (P < .05), respectively. In 2- to 3-month-old SCD/NRF2−/− adult spleen and bone marrow cells, the γ:(γ+βS) ratio was decreased by 29% to 33% (P < .05). As further evidence of NRF2 involvement in globin gene regulation, SCD/NRF2−/− bone marrow hematopoietic progenitors showed a decrease in HbF-expressing cells (F-cells) from 3.2% to 1.2% (P < .05), however, expression of the differentiation markers CD71, Sca1, and c-Kit were not changed significantly (Figure 1C).
NRF2 loss decreased γ-globin gene transcription in SCD mice during gestation. SCD mice with homozygous NRF2 knockout (SCD/NRF2−/−) were established by crossbreeding Townes SCD mice13 and NRF2 knockout (NRF2−/−) mice14 (supplemental Methods; supplemental Table 1). (A) Hematological indices of 2- to 3-month-old SCD/NRF2+/+ (red bars) and SCD/NRF2−/− mice (blue bars) were analyzed by automated complete blood counts and differentials. (B) Expression of human γ-globin and adult βS-globin genes was monitored by qRT-PCR in embryonic day 13.5 and embryonic day 18.5 fetal livers and adult spleen and bone marrow (n = 6-8 mice per group); the γ/(γ+βS) mRNA ratios were calculated. Red circles, SCD/NRF2+/+ mice; blue circles, SCD/NRF2−/− mice. (C) Bone marrow hematopoietic progenitors were isolated from transgenic mice and used for analysis of the different proteins by flow cytometry (supplemental Methods). Representative scatter plots are shown. (D) The levels of HbF and HbS were determined by western blot analysis of whole-tissue protein extracts isolated from adult SCD/NRF2+/+ mice (red bars) and SCD/NRF2−/− mice (blue bars) spleens (n = 3) (top), and quantitative data were generated (bottom); β-actin was used as a loading control. (E) Flow cytometry analysis determined the percentage of HbF-positive RBCs (F-cells) in the peripheral blood stained with fluorescein isothiocyanate–conjugated HbF antibody; RBC gating was performed as described in supplemental Methods (supplemental Figure 2). Representative dot plots for F-cell distribution are shown (left), and the percentage of F-cells was quantified (right) (n = 8). Red circles, SCD/NRF2+/+mice; blue circles, SCD/NRF2−/− mice. C57BL/6 mice were non–sickle cell controls. (F) The level of RBC sickling in the peripheral blood was quantified under normoxic (21% O2) and hypoxic (1% O2) oxygen conditions (left); data represent the mean ± standard deviation (n = 6-7). Representative images under bright field are shown at ×600 magnification (right). *P < .05. qRT-PCR, quantitative reverse transcription polymerase chain reaction.
NRF2 loss decreased γ-globin gene transcription in SCD mice during gestation. SCD mice with homozygous NRF2 knockout (SCD/NRF2−/−) were established by crossbreeding Townes SCD mice13 and NRF2 knockout (NRF2−/−) mice14 (supplemental Methods; supplemental Table 1). (A) Hematological indices of 2- to 3-month-old SCD/NRF2+/+ (red bars) and SCD/NRF2−/− mice (blue bars) were analyzed by automated complete blood counts and differentials. (B) Expression of human γ-globin and adult βS-globin genes was monitored by qRT-PCR in embryonic day 13.5 and embryonic day 18.5 fetal livers and adult spleen and bone marrow (n = 6-8 mice per group); the γ/(γ+βS) mRNA ratios were calculated. Red circles, SCD/NRF2+/+ mice; blue circles, SCD/NRF2−/− mice. (C) Bone marrow hematopoietic progenitors were isolated from transgenic mice and used for analysis of the different proteins by flow cytometry (supplemental Methods). Representative scatter plots are shown. (D) The levels of HbF and HbS were determined by western blot analysis of whole-tissue protein extracts isolated from adult SCD/NRF2+/+ mice (red bars) and SCD/NRF2−/− mice (blue bars) spleens (n = 3) (top), and quantitative data were generated (bottom); β-actin was used as a loading control. (E) Flow cytometry analysis determined the percentage of HbF-positive RBCs (F-cells) in the peripheral blood stained with fluorescein isothiocyanate–conjugated HbF antibody; RBC gating was performed as described in supplemental Methods (supplemental Figure 2). Representative dot plots for F-cell distribution are shown (left), and the percentage of F-cells was quantified (right) (n = 8). Red circles, SCD/NRF2+/+mice; blue circles, SCD/NRF2−/− mice. C57BL/6 mice were non–sickle cell controls. (F) The level of RBC sickling in the peripheral blood was quantified under normoxic (21% O2) and hypoxic (1% O2) oxygen conditions (left); data represent the mean ± standard deviation (n = 6-7). Representative images under bright field are shown at ×600 magnification (right). *P < .05. qRT-PCR, quantitative reverse transcription polymerase chain reaction.
Because the spleen is a primary site for hematopoiesis in SCD,15 we performed globin gene analysis in adult SCD/NRF2−/− spleens. Reflective of γ-globin silencing during gestation, we observed a 73% (P < .05) decrease in HbF expression (Figure 1D). In parallel, F-cell levels were decreased from 2.5% to 1.4% (P < .05) (Figure 1E). Interestingly, HbS levels were unchanged, suggesting that NRF2 does not regulate the adult βS-globin gene and confirming our recent findings that NRF2 preferentially regulated the γ-globin gene in human erythroid progenitors.7 To support a functional role of NRF2, we next determined if the decrease in HbF affected the propensity of RBCs to sickle. Under normal oxygen conditions, NRF2−/− had no effect on RBC sickling (Figure 1F), however, under 1% O2 conditions, we observed 54% and 39% sickling in SCD/NRF2−/− and SCD/NRF2+/+ mice, respectively (P < .05). The regulation of γ-globin expression by NRF2 in SCD mice supports our findings that NRF2 modulates chromatin structure in the β-locus.7
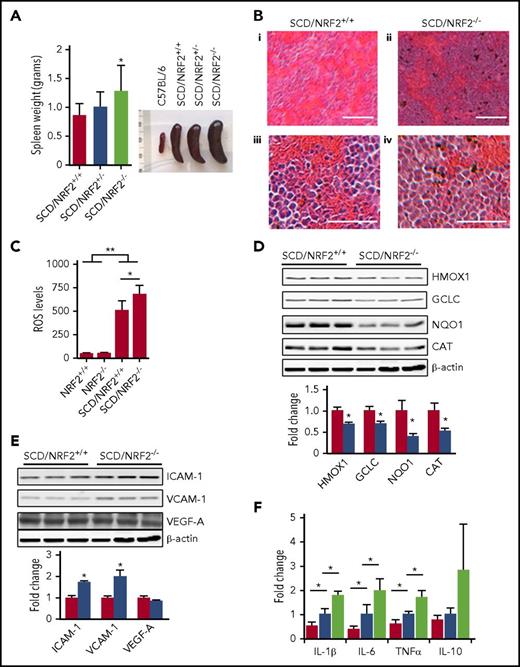
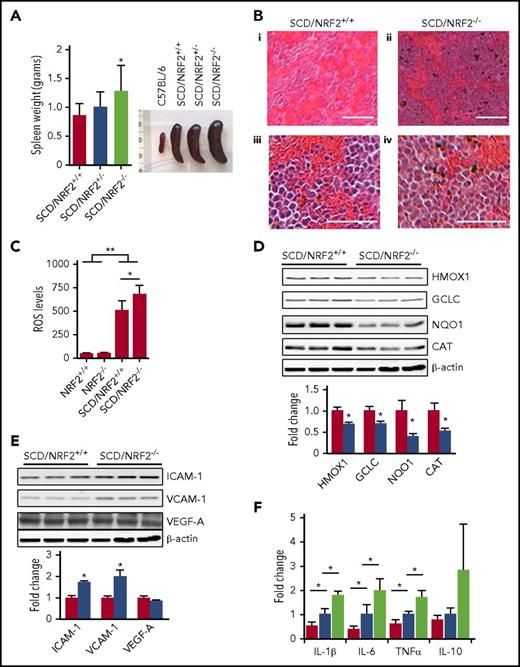
In SCD mice, the spleen is enlarged due to compensatory hematopoiesis, chronic hemolysis, and oxidative stress.16 The loss of NRF2 function in SCD/NRF2−/− mice exaggerated splenomegaly (Figure 2A), supporting the profound effect of oxidative damage with NRF2 loss. To explore the role of NRF2 in the pathophysiology of SCD, tissue morphology was assessed, which revealed expansion of the splenic red pulp and obscuring of the architecture in SCD/NRF2−/− mice. Although the spleens of SCD/NRF2+/+ mice showed expansion of red pulp, the architecture remained intact (Figure 2B).
Loss of NRF2 function produced severe splenomegaly and inflammation in SCD mice. (A) The weight (left) and size (right) of spleens from NRF2 wild-type (+/+), heterozygote (+/−), and knockout (−/−) SCD mice were determined. (B) Histologic section of spleen tissue stained with hematoxylin and eosin is shown; scale bars: (i and ii), 100 µm; (iii and iv), 40 µm. (C) Using 2'-7'-dichlorodihydrofluorescein diacetate staining and flow cytometry, ROS levels in RBCs were determined (n ≥ 6); RBC gating was performed as described in supplemental Methods (supplemental Figure 2). (D) Western blot was completed to determine the expression of antioxidant proteins in spleen whole-tissue extracts for adult SCD/NRF2+/+ mice (red bars) and SCD/NRF2−/− mice (blue bars) (n = 3 per group) (top), and quantitative data were generated (bottom); β-actin was used as a loading control. (E) Western blot was completed as in panel D for the following: intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule (VCAM-1), and vascular endothelial growth factor (VEGF-A) (top), and quantitative data analyzed (bottom). (F) The mRNA levels of the various genes shown were measured by qRT-PCR in spleen cells isolated from 2- to 3-month-old mice (n = 6 per group). Red bars, C57BL/6 mice; blue bars, SCD/NRF2+/+ mice; light green bars, SCD/NRF2−/− mice. The level of mouse 18s ribosomal RNA was used as an internal control. **P < .01; *P < .05. CAT, catalase; GCLC, γ-glutamylcysteine ligase catalytic subunit; HMOX1, heme oxygenase 1; NQO1, NAD(P)H quinone dehydrogenase 1; qRT-PCR, quantitative reverse transcription polymerase chain reaction.
Loss of NRF2 function produced severe splenomegaly and inflammation in SCD mice. (A) The weight (left) and size (right) of spleens from NRF2 wild-type (+/+), heterozygote (+/−), and knockout (−/−) SCD mice were determined. (B) Histologic section of spleen tissue stained with hematoxylin and eosin is shown; scale bars: (i and ii), 100 µm; (iii and iv), 40 µm. (C) Using 2'-7'-dichlorodihydrofluorescein diacetate staining and flow cytometry, ROS levels in RBCs were determined (n ≥ 6); RBC gating was performed as described in supplemental Methods (supplemental Figure 2). (D) Western blot was completed to determine the expression of antioxidant proteins in spleen whole-tissue extracts for adult SCD/NRF2+/+ mice (red bars) and SCD/NRF2−/− mice (blue bars) (n = 3 per group) (top), and quantitative data were generated (bottom); β-actin was used as a loading control. (E) Western blot was completed as in panel D for the following: intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule (VCAM-1), and vascular endothelial growth factor (VEGF-A) (top), and quantitative data analyzed (bottom). (F) The mRNA levels of the various genes shown were measured by qRT-PCR in spleen cells isolated from 2- to 3-month-old mice (n = 6 per group). Red bars, C57BL/6 mice; blue bars, SCD/NRF2+/+ mice; light green bars, SCD/NRF2−/− mice. The level of mouse 18s ribosomal RNA was used as an internal control. **P < .01; *P < .05. CAT, catalase; GCLC, γ-glutamylcysteine ligase catalytic subunit; HMOX1, heme oxygenase 1; NQO1, NAD(P)H quinone dehydrogenase 1; qRT-PCR, quantitative reverse transcription polymerase chain reaction.
It is known that oxidative stress contributes to the clinical severity of SCD.2 Although NRF2 is the master regulator of the oxidative stress response,3 we observed low erythrocyte ROS levels in C57BL6/NRF2−/− mice on a nonhemolytic background (Figure 2C), suggesting that other proteins control oxidative stress. By contrast, significantly higher ROS levels were observed in SCD/NRF2+/+ mice (P < .01), which increased further with NRF2 loss (P < .05), supporting a more prominent role of NRF2 in a chronic hemolysis phenotype. To maintain the oxidative stress response, NRF2 regulates several antioxidative proteins.9-11,17,18 In particular, the levels of HMOX1, NQO1, GCLC, and catalase were decreased by 31%, 30%, 60%, and 48%, respectively (P < .05) in SCD/NRF2−/− mice (Figure 2D). High ROS level combined with free heme produce a detrimental effect, promoting RBC adhesion and vaso-occlusion in SCD.2 Therefore, we determined ICAM-1, VCAM-1, and VEGF expression. Whereas VEGF protein was not changed, ICAM-1 and VCAM-1 levels were increased 1.7-fold and 2.0-fold (P < .05), respectively, in SCD/NRF2−/− adult spleen cells (Figure 2E), suggesting that NRF2 loss would promote adhesion. Additional studies will determine whether ICAM-1 and VCAM-1 are direct targets of NRF2.
Chronic inflammation contributes to the clinical severity of SCD2,19,20 ; therefore, we investigated the proinflammatory molecules interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) in adult spleen cells. The mRNA levels of these genes were higher in SCD mice compared with C57BL/6 mice; strikingly, IL-1β, IL-6, and TNF-α levels increased further in SCD/NRF2−/− mice (Figure 2F). By contrast, expression of the anti-inflammatory cytokine IL-10 was not changed significantly (Figure 2F).
Previous studies have demonstrated that genetic and pharmacologic NRF2 activation mediates antiadhesion and anti-inflammatory effects in SCD mice.9-11 We recently confirmed similar outcomes after chronic treatment with the NRF2 activator dimethyl fumarate.12 Another attractive function of NRF2 is directing l-glutamine into glutathione synthesis.21,22 Because l-glutamine levels are reduced in SCD,23 oral therapy improves the redox potential of sickle RBCs24 and reduces adhesion to human umbilical vein endothelial cells.25 In a recently completed clinical trial, Endari (l-glutamine) was shown to reduce oxidative stress and pain episodes in SCD patients, leading to Food and Drug Administration approval.
Although pharmacologic NRF2 activation provides insights into its protective functions, genetic knockout is required to confirm a role in globin gene regulation during development. Our SCD/NRF2−/− mouse model established the requirement of NRF2 for γ-globin gene transcription during the embryonic and adult gestational stages of erythropoiesis and confirmed γ-globin silencing in the absence of NRF2 expression. Furthermore, NRF2 knockout on a hemolytic background discovered a protection role of NRF2 in the survival of SCD mice during gestation and the perinatal period. Additionally, we observed severe spleen damage, RBC sickling, and increased expression of proinflammatory and adhesion molecules in SCD/NRF2−/− mice. Questions beyond those addressed in this study, such as the role of NRF2 in protecting against severe complications of SCD, including acute chest syndrome, stroke, and kidney disease, among others, can be answered using the SCD/NRF2−/− model, thus expanding its usefulness in the field. Our findings herein combined with published data8-12,22-25 demonstrate the protective role of NRF2 in SCD and support the development of small-molecule activators of NRF2 for the treatment of β-hemoglobinopathies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Masayuki Yamamoto for contributing the NRF2−/− mice. The authors also thank Dorothy Tuan, Hongyan Xu, and Huidong Shi for their helpful insights and discussions of data interpretation.
This work was supported by training grant HL117684 from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (X.Z.); R01 grant HL069234 (B.S.P.) from the NIH, National Heart, Lung, and Blood Institute; and R01 grant NS101967 (B.T.) from the NIH, National Institute of Neurological Disorders and Stroke.
Authorship
Contribution: X.Z. designed and performed the research studies, analyzed the data, and wrote the manuscript; C.X. performed the research studies and analyzed the data; B.T. contributed to the research design, data analysis, and manuscript writing; and B.S.P. contributed to the research design, data analysis, manuscript writing, and supervision of the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xingguo Zhu, Department of Pediatrics, Augusta University, 1120 15th St, CN-4116, Augusta, GA 30912; e-mail: xzhu@augusta.edu.