Abstract
B-cell lymphoma 2 (BCL-2) was discovered at the breakpoint of the t(14;18) in follicular lymphoma >30 years ago. Although inhibition of BCL-2 first proved valuable in lymphoid malignancies, clinical progress in myeloid malignancies lagged. Here, we summarize the basic biology and preclinical results that spurred clinical BCL-2 inhibition in acute myeloid leukemia (AML). Response rates and toxicity for venetoclax in combination with standard AML agents, such as azacitidine, decitabine, and low-dose cytarabine, compare favorably with conventional induction chemotherapy. Durability of response requires further study.
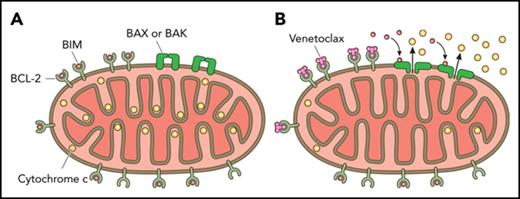
BCL-2 blocks the mitochondrial permeabilization that leads to apoptosis
The B-cell lymphoma 2 (BCL-2) family of proteins regulates mitochondrial outer membrane permeabilization (MOMP), effectively the point of no return in the commitment to apoptotic cell death.1 BCL-2 and related antiapoptotic proteins of this family, including BCL-XL and myeloid cell leukemia sequence 1 (MCL-1), inhibit MOMP by binding and sequestering proapoptotic family members required for MOMP. Certain proapoptotic family members, called sensitizer BCL-2 homology 3 (BH3)-only proteins, act as selective antagonists of antiapoptotic proteins via binding of their BH3 domains into a hydrophobic pocket in antiapoptotic proteins. As we will discuss below, pharmacologic antagonism of BCL-2 has succeeded in the form of “BH3 mimetic” small molecules that antagonize antiapoptotic function by binding the same pocket.
Targeting BCL-2
Since BCL-2 was identified as a protein that kept cells alive, many became interested in targeting BCL-2 to kill cancer cells. First attempts focused on antisense oligonucleotides. However, Bcl-2 represents a challenging antisense target because of its long protein half-life.2 In clinical trials of Bcl-2 antisense G3139 in acute myeloid leukemia (AML) and acute lymphoblastic leukemia, reduction in Bcl-2 expression was inconsistent and of insufficient depth.3 Nonetheless, elimination of BCL-2 via a switchable allele caused dramatic responses in a murine leukemia model.4 This result supported the concept that loss of BCL-2 function by itself could be enough to eliminate cancer cells in vivo, which supported further development of small molecules to antagonize BCL-2.
Subsequent, and more successful, efforts focused on the development of small molecules that mimic the BH3 domain found in all proapoptotic BCL-2 family proteins. The BH3 domain is an amphipathic α-helix that binds a hydrophobic groove on antiapoptotic Bcl-2 family members, negating their cytoprotective activity.5 Several independent efforts identified antagonists of BCL-2 via small molecule screens for compounds that could displace synthetic oligopeptides derived from natural BH3 domains from recombinant BCL-2.6,7 These efforts identified several compounds of relatively weak inhibitory constant (Ki > 100 nM) affinity, none of which met with clinical success because of problems with off-target toxicity and relatively low on-target activity.8 In a modification of this strategy, scientists at AbbVie identified compounds that bound at 2 distinct sites in BCL-2’s BH3 binding groove.9 These 2 moieties were then chemically connected, resulting in a significantly larger compound with much greater affinity for the groove. Following a structure-activity relationship by nuclear magnetic resonance (“SAR by NMR“) strategy, ABT-737 was the first high-affinity inhibitor of BCL-2 to emerge. The development of a high-affinity protein–protein interaction inhibitor that directly activated apoptosis represented a landmark event in medicinal chemistry, as well as in the cell death field.
ABT-737 binds Bcl-2, Bcl-XL, and Bcl-w with high affinity (Ki < 1 nM), but it binds weakly (Ki > 460 nM) to other antiapoptotic Bcl-2 family members, including MCL-1 and BFL-1.9 Preclinical studies demonstrated its ability to kill cells, including primary patient cancer cells. However, poor pharmacologic properties prevented clinical development. An orally bioavailable derivative of ABT-737, navitoclax (formerly ABT-263), showed activity in chronic lymphocytic leukemia (CLL) and small-cell lung cancer10,11 in early clinical trials. However, it caused on-target dose-limiting thrombocytopenia because platelets are dependent on the antiapoptotic protein BCL-XL for their survival.12-14 Although very little clinically significant bleeding was observed, numerical thrombocytopenia limited the use of this agent in many cancers, including AML. This prompted AbbVie’s development of ABT-199 (venetoclax), a highly selective small molecule BH3 mimetic with even greater affinity (Ki < 1 nM) for BCL-2 but much lower affinity (Ki > 100 nM) for BCL-XL.15
Initial clinical studies focused on CLL and non-Hodgkin lymphoma.16-18 The remarkable activity in CLL as a single agent, followed by even better activity in combination with other agents active in CLL, have yielded several US Food and Drug Administration (FDA) approvals in CLL, with many trials in lymphoid malignancies ongoing. Progress in myeloid malignancies has lagged, however. This lag might be attributed to the fact that BCL-2 was first identified in a lymphoid cell,19 whereas MCL-1 was first identified in a myeloid cell.20 This may have yielded the unfortunate assumption that because lymphoid malignancies were often dependent on BCL-2, then myeloid malignancies would be dependent on MCL-1 but not BCL-2. Although there is a superficially attractive symmetry in this concept, it is based on mere accidents of discovery and naming of the proteins rather than on data or biological principles. Inhibition of MCL-1 in AML is only beginning to be explored clinically, with efficacy and toxicity yet to be established (Table 1). However, clinical results over the last few years already offer compelling evidence that inhibition of BCL-2 is likely to offer a very valuable new agent for the treatment of AML.
Therapeutic index
Before proceeding to AML, it is worth considering the molecular biology of the efficacy of BCL-2 inhibition, how this permits identification of sensitive cells, and how it explains the existence of a therapeutic index.
It is not immediately obvious that there should be a therapeutic index for a BCL-2 inhibitor. Plenty of cancer cells express BCL-2, and plenty of normal cells do, too. Moreover, superficially, one might expect a cell expressing low levels of BCL-2 to be even more sensitive to BCL-2 inhibitor than one expressing high levels, because there is simply less to inhibit. In fact, levels of BCL-2 by itself do not provide a good explanation for sensitivity to BCL-2 inhibition. Instead, it is the quantity of BCL-2 that is binding and sequestering proapoptotics like BIM or BAX that matters.21-23 We call this “primed” BCL-2, and how it functions to determine sensitivity to BCL-2 inhibition is explained in Figure 1. The more primed BCL-2 there is in a mitochondrion, the more sensitive the cell bearing that mitochondrion will be to BCL-2 inhibition.24
The differential response of primed vs unprimed mitochondria to a BCL-2 selective BH3 mimetic. In the case of a primed cell (top), BCL-2-bound-BIM (or activated BAX) can be displaced by the BCL-2 antagonist to induce BAX or BAK dependent MOMP, irreversibly committing the AML cell to death. In the case of an unprimed cell (bottom), treatment with the BCL-2 antagonist does not induce MOMP due to the lack of proapoptotic proteins prebound to the BCL-2. Reprinted from Potter and Letai24 with permission.
The differential response of primed vs unprimed mitochondria to a BCL-2 selective BH3 mimetic. In the case of a primed cell (top), BCL-2-bound-BIM (or activated BAX) can be displaced by the BCL-2 antagonist to induce BAX or BAK dependent MOMP, irreversibly committing the AML cell to death. In the case of an unprimed cell (bottom), treatment with the BCL-2 antagonist does not induce MOMP due to the lack of proapoptotic proteins prebound to the BCL-2. Reprinted from Potter and Letai24 with permission.
Priming of BCL-2 can be identified by protein-interaction assays like coimmunoprecipitation assays, but it can perhaps be most systematically and conveniently measured by BH3 profiling. In BH3 profiling, mitochondria are exposed to synthetic BH3 peptides, and MOMP is measured by quantifying egress of intermembrane space proteins or potential sensitive dyes. Certain of these peptides selectively antagonize individual antiapoptotic proteins.21 Thus, one can infer dependence on individual antiapoptotic proteins like BCL-2 based on the pattern of induction of MOMP by these peptides.25 BH3 profiling demonstrates that BCL-2 expressed by most normal cells is simply poorly primed by proapoptotic proteins; thus, those cells are not sensitive to BCL-2 inhibition.26,27 Normal mature lymphocytes are an exception, because these generally express BCL-2 that is primed to some extent, likely explaining the lymphopenia that is a common side effect of venetoclax treatment.28,29 In sensitive cancer cells, BCL-2 is heavily primed by proapoptotics: by BIM in CLL and by BIM and BAX in AML.22,30,31 BH3 profiling has proven useful in identifying diseases that are sensitive to BCL-2 inhibition (eg, CLL, acute lymphoblastic leukemia, AML, multiple myeloma, and blastic plasmacytoid dendritic cell neoplasm), as well as for stratifying individual sensitivity within a disease.22,32-38 Although BH3 profiling has heretofore been used purely in a research setting, one of us (A.L.) is pursuing its implementation in clinical practice in a Clinical Laboratory Improvement Amendments–approved setting.
Preclinical data supporting BCL-2 inhibition in AML
Despite early skepticism of the usefulness of BCL-2 inhibition in AML, early evidence based on studies with ABT-737 showed that significant subsets of AML depend on BCL-2 for survival. ABT-737 induced apoptosis as a single agent in AML-derived cell lines, in primary blasts, and in phenotypically defined AML stem cells; inhibited the growth of clonogenic AML progenitor cells; and was effective at reducing the leukemia burden in vivo in a xenograft model of AML.30 Notably, this study demonstrated that AML cells assemble apoptotic machinery, such as Bax and Bim, on the mitochondrial membrane, kept in check by inhibitory Bcl-2 proteins, hence rendering them exquisitely sensitive to the pharmacological blockade of this pathway. ABT-737 neutralizes Bcl-2, freeing up Bax, which spontaneously assumes an active conformation, and Bim, in the milieu of AML mitochondria, resulting in the mitochondrial dysfunction, release of cytochrome c, and activation of caspases that initiate the cell death process. Subsequently, ABT-737 was also found to be effective in an immune-competent murine model of AML.39 Independent studies of the mitochondria of AML cells also supported their dependence on BCL-2.33 BH3 profiling demonstrated widespread dependence on BCL-2 in the mitochondria of primary patient AML myeloblasts. Moreover, dependence on BCL-2 in hematopoietic stem cells was observed to be consistently less, suggesting an exploitable therapeutic index for the inhibition of BCL-2 in AML.
Having supported the importance of BCL-2 inhibition in AML via independent methods, we decided to collaborate on subsequent studies to speed translation to the clinic. We found that ABT-199 induced rapid apoptosis of multiple AML cell lines at nanomolar concentrations and inhibited leukemia progression in an in vivo murine AML xenograft model.34 Importantly, ABT-199 induced apoptosis in a large percentage of chemotherapy-sensitive and chemorefractory primary AML myeloblasts and AML stem/progenitor cells in vitro. AML cytotoxicity inversely correlated with BCL-XL expression and directly correlated with BCL-2 expression and mitochondrial membrane depolarization, indicating on-target BCL-2 inhibition at the mitochondria, and it was largely independent of mutation status. In this study, mitochondrial BH3 profiling predicted responsiveness of AML primary cells to ABT-199 in vitro and in an in vivo patient-derived xenograft model and was proposed as a predictive biomarker of therapeutic response to ABT-199 AML. Together, these findings convinced AbbVie to initiate clinical evaluation of venetoclax in AML.
Clinical trials of BCL-2 inhibition in AML
Venetoclax monotherapy in AML
On the basis of this preclinical data, a phase 2 clinical trial of venetoclax in relapsed/refractory AML (M14-212) was launched in December 2014. A daily ramp-up of venetoclax dosing was implemented to achieve the target venetoclax dose of 800 mg, in conjunction with measures to prevent, monitor, and manage potential tumor lysis syndrome seen in CLL16 ; of note, no clinical tumor lysis syndrome occurred during venetoclax treatment in the AML study. A total of 32 patients was enrolled, with venetoclax administered until disease progression or unacceptable toxicity.35 The median age was 71 years (range, 19-84), and the majority were heavily pretreated, with 41% having received ≥3 prior regimens. A total of 62% had complex or del(7q) poor-risk cytogenetics. Single-agent venetoclax produced an overall response in 5 of 32 relapsed/refractory AML patients (complete response [CR] in 1 patient, CR with incomplete hematologic recovery [CRi] in 4 patients), with additional blast reductions not meeting the response criteria in 19% of patients. The responses were short-lived, with median time to progression of 2.5 months. Escalating the dose of venetoclax to 1200 mg daily at the time of progression or in patients lacking a response at an 800-mg dose did not demonstrate additional activity. Venetoclax therapy was well tolerated, with common toxicities of grade 1/2 gastrointestinal adverse effects, as well as grade 3/4 febrile neutropenia in ∼30% of patients.
BH3 profiling was performed on pretreatment bone marrow biopsies of 12 patients. Although baseline BCL-2 dependence of blast mitochondria correlated with clinical response, a better predictor of response was lack of myeloblast dependence on the antiapoptotic proteins Bcl-XL or MCL-1, known resistance factors to selective Bcl-2 inhibition.35 Analysis of genomic predictors showed hints that mutations in IDH1/IDH2 were associated with higher likelihood of response, whereas FLT3-ITD or PTPN11 mutations were associated with lack of response or progression; however, the patient numbers were insufficient for definitive conclusions.40
Venetoclax combination therapy in AML
Although the single-agent trial demonstrated biological activity and tolerability, limited and short-lived responses to venetoclax as a single agent in relapsed AML prompted the exploration of combination strategies. One strategy has been to combine it with agents already in use in AML, especially those for which efficacy was supported by preclinical data. In preclinical studies, BCL-2 inhibition with ABT-737 or ABT-199 showed additive or synergistic activity with 5-azacytidine, a hypomethylating agent (HMA) approved by the FDA for treatment of myelodysplastic syndrome but also commonly used off-label in the United States to treat AML in the elderly and unfit.41,42 5-Azacitidine was shown to reduce MCL-1 protein levels in blasts, supporting combination with BCL-2–targeting agents.41 Venetoclax also synergistically induced apoptosis when combined with cytarabine and idarubicin in a TP53-dependent manner, although higher doses of idarubicin suppressed MCL-1 and induced cell death independently of TP53.43
In a phase 1b study of venetoclax plus HMAs (5-azacitidine or decitabine) in 145 elderly (≥65 years) patients with newly diagnosed AML who were ineligible for standard induction therapy, the toxicity profile of the combination was similar to that of HMAs alone used as an induction regimen.44 The most common serious treatment-related adverse effects were febrile neutropenia and common grade 3/4 adverse effects related to myelosuppression. No events of tumor lysis syndrome were recorded, and early (30-day) mortality was low (3.4%). The combination was highly active, with a composite CR/CRi rate of 67%, overall response rate of 83%, median overall survival (OS) of 17.5 months, and a 2-year OS estimate of 46%. With these encouraging results, which exceeded historical expectations for 5-azacitidine with composite CR/CRi of 28% and median OS of 10.4 months,45 this combination received FDA breakthrough designation in early 2017; a randomized phase 3 registration study of venetoclax (400 mg/d) plus standard doses of 5-azacitidine vs azacitidine alone in elderly AML patients unfit for induction chemotherapy is accruing (Table 1). Preclinical data support the influence of select genetic alterations, like p53 (negative) or IDH1/2 mutation (positive), on the response to venetoclax. Reported clinical data thus far lack the maturity and patient numbers for a firm statistical comment on the effect of these alterations in the clinic.
The combination of venetoclax (600 mg) with the lose-dose cytarabine (NCT03069352) is currently being evaluated in a randomized phase 3 trial with similar design as for HMA combination. Preliminary results of the completed phase 1/2 combination trial that enrolled 61 patients at the phase 2 dose of venetoclax showed acceptable safety (3% early death rate, 36% grade 3-4 febrile neutropenia) and impressive activity, with CR/CRi rates of 62% and median OS of 11.4 months.46 Response rates were higher among patients with intermediate-risk cytogenetics (CR/CRi, 76%; median OS, 15.7 months) compared with adverse-risk cytogenetics (47%; median OS, 5.7 months) or TP53 mutations (50%; median OS, 6.5 months), and all patients with NPM1 (n = 7) or biallelic CEBPA (n = 3) mutations responded.
Most responses occurred within 2 cycles in both studies. Chemotherapy-induced cytopenias were addressed with venetoclax interruption, delaying the next cycle of chemotherapy until count recovery. Recurrent neutropenia in patients who have achieved CR and full count recovery may warrant shortening the duration of venetoclax administration to ≤3 weeks in a given 28-day cycle. The drug is metabolized by CYP3A, and venetoclax dose should be reduced when strong CYP3A inhibitors, such as azoles, are used. In addition to low-intensity therapy, venetoclax combinations with chemotherapy or targeted agents are ongoing in AML (Table 1). These include combinations with standard or high-doses chemotherapy, such as cytarabine and idarubicin, in younger or elderly fit patients. From targeted agent(s) combinations, venetoclax combined with MDM2 inhibitor idasanutlin showed encouraging activity in an ongoing phase 1b relapsed/refractory study of elderly patients with AML.47 This combination was based on preclinical work demonstrating that activation of p53 through pharmacologic inhibition of MDM2 reduces Ras/Raf/MEK/ERK, resulting in activation of GSK3β and MCL-1 phosphorylation and degradation, hence overcoming resistance to venetoclax.48 Very little is known about molecular mechanisms underlying acquired resistance to venetoclax, although in vitro studies support the possibility of upregulation of antiapoptotic proteins not targeted by venetoclax like MCL-1.49 Other combinations have entered trials, such as bromodomain inhibitor ABBV-075 or TRAIL receptor agonist ABBV-621. Table 1 also summarizes the exciting growth of trials in AML using the growing number of BH3 mimetics targeting BCL-2 or MCL-1 being developed by different companies.
Conclusion
Despite the lack of identified genetic alterations in BCL-2 in AML, targeting of BCL-2 has emerged as an efficacious and well-tolerated clinical strategy in AML, based on preclinical studies founded in myeloblast function rather than genetics. As with most narrowly targeted drugs, the optimal clinical usefulness of BCL-2 inhibitors will likely come in combination with other active agents. Combination candidates will include agents already in use in AML, such as HMAs and low-dose cytarabine. Additionally, there will be opportunities to combine with other targeted agents with activity in AML. A particularly attractive combination yet to be clinically tested in that of a BCL-2 inhibitor and an MCL-1 inhibitor, because BCL-2 and MCL-1 act reciprocally as resistance factors for inhibition of the other. Thus, the simultaneous targeting of both, if proven tolerable, might have great efficacy. Whatever combinations emerge as the most useful in the growing set of clinical trials, it seems certain that BCL-2 inhibitors and other BH3 mimetics have an important future in the treatment of AML and are likely here to stay in this disease.
Acknowledgments
The authors recognize the many people in industry and academia whose important contributions to BH3 mimetic therapy in AML could not be individually recognized due to space constraints. The authors thank Joel Leverson and Jalaja Potluri (AbbVie) for informative discussions on the development of venetoclax in AML.
Authorship
Contribution: M.K. and A.L. developed the ideas and wrote the manuscript.
Conflict-of-interest disclosure: A.L. has performed paid consulting for and has received research support from Astra-Zeneca, Novartis, and AbbVie. M.K. has served as a consultant for AbbVie, Genentech, and F. Hoffmann-La Roche and has received grants from AbbVie, Genentech, Eli Lilly, Cellectis, Calithera, Stemline, Threshold, Flexus Biosciences, and Novartis.
Correspondence: Anthony Letai, Mayer 430, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02052; e-mail: anthony_letai@dfci.harvard.edu.