In this issue of Blood, Sanders et al1 made a very insightful observation about mutational burden in 3 early-onset leukemias that led to the identification of methyl-binding domain 4 (MBD4) as a candidate gene for a new acute myeloid leukemia (AML) predisposition syndrome.
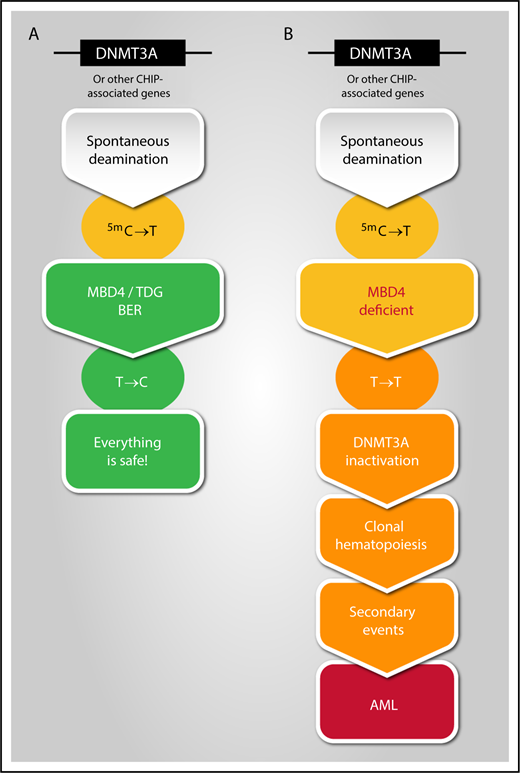
Potential link between spontaneous deamination, MBD4 deficiency, and development of clonal hematopoiesis leading to AML transformation. Illustration by Manuel Buscarlet, Hôpital Maisonneuve-Rosemont, Montréal, Canada.
Potential link between spontaneous deamination, MBD4 deficiency, and development of clonal hematopoiesis leading to AML transformation. Illustration by Manuel Buscarlet, Hôpital Maisonneuve-Rosemont, Montréal, Canada.
CG dinucleotides serve as sites for cytosine modifications, beginning with the addition of a methyl group by DNA methyltransferase (DNMT) enzymes to form 5-methylcytosine (m5C). Spontaneous deamination of m5C results in a mispaired thymine, which if left within the DNA, causes a C>T transition mutation. C>T transitions that occur by this mechanism are a common cause of age- and cancer-associated mutations, because they occur at a rate 10 to 50 higher than other transitions.2 At least 2 proteins are responsible for repairing these unpaired thymine bases and protecting our genome integrity: thymine DNA glycosylate3 and MBD4 (see figure).4 In this paper, the authors measured tumor mutational burden and found 3 cases with a 33-fold higher level of mutations than in sporadic AML, with >95% being C>T transitions that occurred within a CG dinucleotide, suggesting a failure to detect a deaminated m5C base. Importantly, 2 of these cases occurred in sisters who developed AML 4 years apart, and for which the second sister was the apparently healthy peripheral stem cell donor for the first. Deep sequencing studies identified a germline homozygous deletion of MBD4 in 1 patient and compound heterozygous MBD4 mutations in the 2 sisters.
In addition to the high C>T transition mutation burden, the 3 leukemia cases also shared acquired biallelic DNMT3A mutations and either IDH1 or IDH2 hot spot mutations, a relatively rare combination in de novo AML. Interestingly, all of these mutations arose from C>T transition mutations. Analysis of sequential samples in treatment and remission using single-cell sequencing methods demonstrated that (1) DNMT3A mutations occurred first and were present in remission, (2) several DNMT3A clones coexisted, and (3) mutations were also present in other genes associated with clonal hematopoiesis of indeterminate potential (CHIP),5 such as TET2, ASXL1, and TP53. The authors were able to reproduce this signature in an mbd4-deficient mouse model.
Germline predisposition to myeloid neoplasms has recently been recognized by the World Health Organization (WHO).6 This includes clinical bone marrow failure syndromes (eg, Fanconi anemia, Diamond-Blackfan anemia, etc) and mutations in a specific genes (eg, ANKRD26, CEBPA, DDX41, ELANE, ETV6, GATA2, HAX1, MECOM/EVI1, RTEL1, RUNX1, SAMD9, SAMD9L, and SRP72) reviewed in Godley and Shimamura.7 Does MBD4 have all the prerequisites to join this infamous club? Perhaps not yet, given that the WHO requires 2 independent publications to establish a new syndrome. What is the prevalence of MBD4 germline mutation? This should be determined in the normal population of young individuals. It would also be crucial to determine its prevalence in AML across ages, which would allow determining the penetrance of the mutation. However, given that only 9/10 683 (0.8%) of The Cancer Genome Atlas subjects carried monoalleleic germline MBD4 mutations, we can extrapolate that homozygous germline mutations or double heterozygous mutations will be exceedingly rare.
MBD4 deficiency seems to recapitulate, accelerate, and aggravate the CHIP phenotype. The most frequently mutated genes in CHIP are DNMT3A and TET2,8 both of which are associated with increased hematopoietic stem cell self-renewal capacity. The relative risk of transformation to hematological cancers is estimated to be increased by 2.3- to 10-fold. Risk factors for transformation have been identified and include the specific gene, the variant allele frequency of the mutation, and the number of different mutations.9 The 3 MBD4-deficient patients had a high frequency of different DNMT3A mutations, acquisition of new mutations, and rapid transformation, indicating that acquired mutation in DNMT3A, which supports clonal expansion, is an early and essential step in AML progression.
In contrast to MBD4 germline inactivation, CHIP is common and may be responsible for a significant proportion of age-associated AML. Is it possible that acquired dysfunction in MBD4 or in other elements of the base excision repair pathway occurs with aging and is an initiator of CHIP physiopathology? This certainly merits further investigation. Finally, the diagnosis of AML after donation of hematopoietic stem cells for a sibling hematopoietic stem cell transplant highlights how important it is to consider germline predisposition syndromes at the time of related donor.10 In this case, the recognition of a unique situation, AMLs diagnosed in siblings with an unusual mutational signature, has led to the recognition of a new familial germline myeloid malignancy syndrome, an observation that will help better understand the role of dysregulated epigenetic marks in the development of acute leukemia.
Conflict-of-interest disclosure: The authors declare no competing financial interests.