In the day-to-day practice of hematology, we are often asked to comment on seemingly innocuous findings such as a low neutrophil count. With the evolution of the modern genetic age, we have gained the ability to provide explanations for pathophysiological findings, thus adding to the base of knowledge regarding hematopoiesis. In this issue of Blood, Magoulas et al add to our knowledge with their thorough analysis of a rare consanguineous family, proving again the utility of studying rare disease for the benefit of wider biologic knowledge.1 We have thus gained a deeper understanding of myelofibrosis (MF), a disease found mostly in older people, by studying it in the young.
MF is generally a variant of myeloproliferative neoplasms that affects mainly those older than 50 years and is associated primarily with mutations in genes related to myeloproliferative neoplasms such as JAK2, MPL, and CALR.2 Approximately 10% of MF cases have no definable genetic cause. In pediatrics, MF is an extremely rare disease, and cases typically culminate in leukemia.3 The case presented by Magoulas et al is marked by the presence of MF in the context of multiple congenital abnormalities, including severe neurocognitive dysfunction. This serves as a reminder that although the most appreciable part of the phenotype is hematologic, the defective protein function involved plays a role within many tissues throughout the body; indeed, before the Magoulas et al letter was published, reports showed that RBSN mutations had a relationship with severe physical and neurocognitive abnormalities.4
Haploinsufficiency is suggested as a cause of other blood cell abnormalities in RBSN carriers in the family presented by Magoulas et al. The unaffected child and both parents exhibited blood count abnormalities, and those of us who are hematologists can easily envision encountering innocuous cases and cases with milder manifestations many times throughout our careers. This is a reminder that family history can provide important clues to understanding the etiology of the abnormality being evaluated and also that rare disorders are not always so rare.
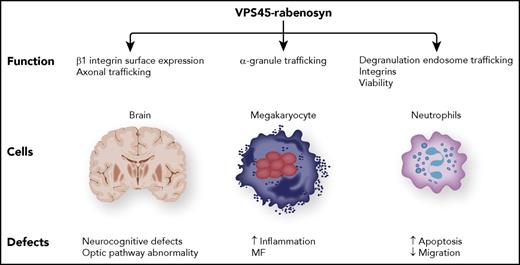
The putative binding partner of rabenosyn (RBSN) is VPS45, in which mutations have been shown to be a cause of MF, severe congenital neutropenia, and severe infection.5-8 Studies of VPS45 have solidified the role of the entire protein complex in protein trafficking via endosomes (see figure). In granulocytes, such protein transport entails the proper localization of granules that bind and cause the death of engulfed bacteria. Such a mechanism can be extrapolated to hematopoietic cells as in the regulation of proinflammatory proteins in megakaryocytes implicated in the primary formation of MF. As a reminder that these proteins are systemic, patients with MF exhibit profound neurocognitive developmental defects.
In sum, it should not be surprising that RBSN mutations result in primary MF, given the previous findings from the analysis of VPS45. Epistasis of RBSN is confirmed by the phenocopy of its mutant phenotype being so consistently similar to that of VPS45. Such established biochemistry can enable the rapid confirmation of observed DNA sequencing data without further validation.
Conflict-of-interest disclosure: The author declares no competing financial interests.