Abstract
Introduction: Patients (pts) with histologically aggressive MCL (HA-MCL; blastoid or pleomorphic) including [de novo (dnMCL) or those transformed from classic morphology (t-MCL)], exhibit a poor prognosis. This analysis provides a comprehensive assessment of so far the largest patient cohort with HA-MCL treated with various modalities.
Methods: We included all HA-MCL pts [blastoid (n=142) or pleomorphic morphology (n=26)] at MD Anderson Cancer Center from 12/1997 to 07/2018. Among the 168 pts, 99 were dn-MCL and 69 were t-MCL. Pt characteristics were collected at the time of initial diagnosis in dnMCL and at transformation in t-MCL. Overall survival (OS) was defined from the time of initial diagnosis of HA-MCL to death/last follow-up and failure free survival (FFS) - time of starting first-line treatment after diagnosis of HA-MCL to treatment failure. Whole-exome sequencing (WES) with SureSelect Human All Exon V6 was performed on specimens from 100 pts (among them, 27 tumor-normal pairs and 73 tumors without matched germline), this included CNT (classic never transformed=52), dnMCL=27, t-MCL=21. Two t-MCL pts had tumors sequenced at both classic and HA-MCL phase.
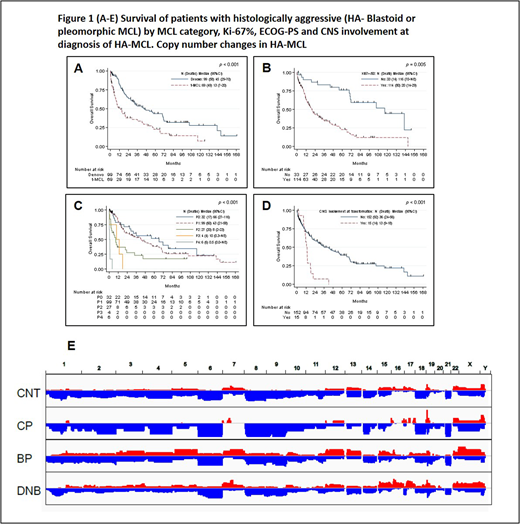
Results: Median age for all 168 pts was 65 years (range, 39 to 95). Median time from initial diagnosis of classic t-MCL to HA t-MCL was 39 months (5 to 240 months). The median follow up after the diagnosis of HA-MCL was 19 months (0.1-168). Main clinical features of HA-MCL were - 72% pts had stage IV disease, 67% with marrow involvement, 27% leukemic phase, 20% with B symptoms, 6% had ECOG PS (3-4) and central nervous system involvement in 9% pts. Other features were, median Ki-67% 70% (10-100%), complex karyotype 11%, LDH > upper limit of normal 44%, Sox-11 positive in 82%, median β2M of 2.9 mg/dL. In pt subsets, t-MCL were distinct from dnMCL in having significantly higher median age, higher Ki-67% and lower proportion of marrow involvement at diagnosis. All pts with t-MCL had prior treatment for MCL before transformation. Overall, the median OS after diagnosis of HA-MCL was 32.5 months (45 and 13 months for dnMCL and t-MCL respectively; p=0.001) and the median FFS was 12.5 months (22 and 5 months for dnMCL and t-MCL respectively; p=0.001). In univariate analysis, factors significantly associated with inferior OS in HA-MCL were older age, high Ki-67%, higher LDH, elevated WBC count, higher β2M, lower hemoglobin, lower platelet counts and advanced ECOG-PS, presence of B symptoms, CNS involvement, complex karyotype and t-MCL. Recursive partitioning analysis revealed that Ki-67% ≥50%, LDH ≥1282, β2M ≥ 4, hemoglobin <12 and platelet count <58,000 were associated with significantly increased risk of death. In multivariate analysis (MVA), adjusting for the above variables, higher age (yrs), t-MCL category, Ki-67% ≥50%, ECOG-PS (1-4 compared to 0) and CNS involvement were significantly associated with inferior OS in HA-MCL. (Figure-1 A-D) For FFS, presence of t-MCL, poor ECOG-PS, LDH ≥ 663, CNS involvement were predictive of inferior FFS by MVA. Pts who received ibrutinib based therapies as their first line treatment for HA-MCL had longer FFS compared to intensive chemoimmunotherapy such as R-HCVAD; HR 0.40 (95%CI 0.16-0.99; p=0.04).
Frequently mutated genes in HA-MCL were NF1 (34%), SDHA (34%), TP53 (31%), NOTCH1/2 (31%), ATM (26%), and KMT2D (23%). Tumors with different histology subtypes exhibited distinct mutation profiles. TP53 mutations were frequently seen in dnMCL than in t-MCL (39% vs. 20%) and rare in CNT (5%, p=0.0015, compared to dnMCL). SDHA was frequently mutated in dnMCL than in t-MCL or CNT (48% vs. 10% vs. 3%, p<0.01). Deleterious ATM and FAT2 mutation were frequent in HA t-MCL than in classic phase of t-MCL (30% vs. 0%, p=0.2 and 40% vs. 0% respectively, p=0.2). Copy number analysis revealed similar pattern between CNT and classic phase t-MCL (except 19p gain which is 2x higher frequency in classic phase t-MCL than CNT), but distinct copy number gains were noted in HA t-MCL and dnMCL (Fig. 1E). Striking difference between dnMCL and HA t-MCL was observed on chromosome 17, with much higher levels of 17p loss and 17q gain in dnMCL than in HA t-MCL.
Conclusions: Histologically aggressive MCL (dn or t-MCL) is a therapeutic challenge. Pts with t-MCL, Ki-67% ≥ 50%, poor PS and CNS involvement had the worst outcomes. Ibrutinib based treatments improve the outcomes of HA-MCL. Further comprehensive molecular analyses will be reported by our group.
Nastoupil:Spectrum: Honoraria; Janssen: Research Funding; Novartis: Honoraria; Merck: Honoraria, Research Funding; Juno: Honoraria; Gilead: Honoraria; Karus: Research Funding; TG Therappeutics: Research Funding; Genentech: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Champlin:Sanofi: Research Funding; Otsuka: Research Funding. Neelapu:Novartis: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Research Funding; Acerta: Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Poseida: Research Funding; Cellectis: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karus: Research Funding; Unum Therapeutics: Membership on an entity's Board of Directors or advisory committees. Fowler:Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy, Research Funding. Wang:MoreHealth: Consultancy; Kite Pharma: Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Research Funding; Acerta Pharma: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Research Funding; Dava Oncology: Honoraria; Pharmacyclics: Honoraria, Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.