Abstract
Background: In chronic myeloid leukemia (CML), deep molecular responses improve long-term prognosis, and patients (pt) with sustained MR4.5 are candidates for treatment discontinuation. Hence, eliminating minimal residual disease (MRD) in CML is an important goal of tyrosine kinase inhibitor (TKI) therapy. JAK-STAT signaling is constitutively activated in CML. Preclinical studies have shown JAK2 inhibition to induce apoptosis of CML stem cells. Ruxolitinib (RUX) is a potent, selective inhibitor of JAK1/2 which has shown activity in myeloproliferative neoplasms. We hypothesized that adding ruxolitinib to ongoing TKI therapy may improve the depth of response.
Methods: This was a phase I/II open label single center study. We enrolled pts ≥18 yrs with BCR/ABL-positive CML on continuous imatinib (IM) therapy for at least 18 months (mo) and stable dose for 6 mo with complete cytogenetic response (CCyR). Pts needed to have detectable BCR-ABL transcripts by polymerase chain reaction (PCR), either had never achieved major molecular response (MMR), or lost MMR with 1-log fold rise in PCR, or lacked sustained MMR despite ≥2 years of TKI therapy, or lacked sustained (≥2 years) "complete molecular response" (CMR, defined as undetectable BCR-ABL PCR with at least 100,000 abl copies detected) despite ≥5 years on TKI. BCR-ABL transcripts were tested using quantitative real-time reverse-transcriptase PCR. Pts needed to have ECOG score ≤2 and adequate organ function. For the phase I portion, pts only needed to be in complete hematologic response, only pts on IM were eligible and those with history of blast phase (BP) were excluded. Dose escalation was done using a "3+3" design. Pts could continue therapy with RUX for up to 3 years. The primary objectives were to determine dose-limiting toxicities (DLT) and maximum tolerated dose (MTD) of the combination of IM and RUX, and to determine the clinical activity of the combination. Secondary objectives were to determine safety of the combination, rates of one-log reduction of BCR-ABL PCR from baseline or CMR, and survival outcomes including overall survival (OS), event-free survival (EFS), and survival free from blastic transformation.
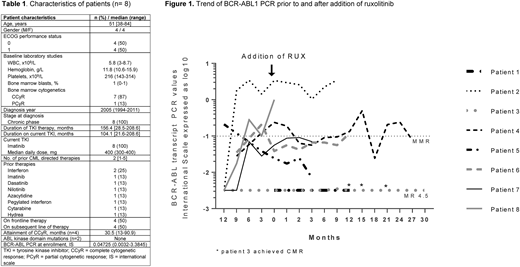
Results: Between November 2013 and July 2018 we enrolled 8 pts in the phase I portion of the study. The pt characteristics are shown in table 1. 1 pt did not have sustained CMR and the other 7 pts had increasing PCR which prompted enrollment. The median baseline BCR-ABL at enrollment was 0.0473 IS (range 0.0032-1). 1 pt is too early for assessment. The remaining 7 pts had been followed-up for a median of 46.6 mo (range 9.5-57.1) and had received RUX for a median of 12.4 mo (range 4.6-37.1). RUX dosing was 5 mg BID (dose level 0), 10 mg BID (dose level 1) and 15 mg BID (dose level 2) for 3, 3 and 2 pts, respectively. The median PCR at the end of study was 0.07 IS (range undetectable-3.4895, fig. 1) There were no DLTs. The MTD of RUX in combination with IM is 15 mg twice daily. There have been 39 treatment-emergent adverse events (TEAEs) in 6 pts with 10 grade 3/4 TEAEs. The most common TEAEs were infections (23%) and anemia (8%). The infections comprised 3 cases of pneumonia (unknown pathogen), 2 cases of upper respiratory tract infection, 1 case of E. coli sepsis, 1 case of shingles, and 1 case of gastroenteritis. There was 1 grade 5 TEAE of cardiac arrest unrelated to the study regimen. There were 10 unique serious AEs in 4 pts, all unrelated to study drug. 1 pt who started treatment with MR4.5 achieved intermittent CMR 18 mo into therapy. 1 pt who started treatment with PCyR after being on 2nd line TKI for 21.6 mo achieved CCyR 6 mo into therapy. No pt achieved the other response criteria of 1-log fold BCR-ABL reduction (fig. 1). No pt progressed to BP. Median OS and EFS has not been reached (NR, range 52.7 mo-NR). 7 pts have discontinued and 1 pt was recently started on therapy. Reasons for treatment discontinuation include completion of therapy in 2 pts, lack of response in 2 pts, withdrawal of consent in 2 pts, and death in 1 pt (due to cardiac arrest unrelated to study regimen). The pt who died had never achieved CCyR despite 28.5 mo on TKI and had never achieved MMR.
Conclusion: It is feasible to combine RUX with IM for pts with persistent minimal residual CML while receiving TKI. There were no DLTs with RUX 15 mg BID and IM. However, in this small cohort of pts, the combination of RUX and IM did not have any significant effect on reducing MRD. The trial is being amended to include pts receiving other TKI.
Maiti:Celgene Corporation: Other: Research funding to the institution. Jabbour:Novartis: Research Funding; Takeda: Consultancy, Research Funding; Abbvie: Research Funding; Pfizer: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding. Ravandi:Jazz: Honoraria; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Xencor: Research Funding; Sunesis: Honoraria; Sunesis: Honoraria; Orsenix: Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Seattle Genetics: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Macrogenix: Honoraria, Research Funding; Orsenix: Honoraria; Astellas Pharmaceuticals: Consultancy, Honoraria; Abbvie: Research Funding; Macrogenix: Honoraria, Research Funding; Xencor: Research Funding; Amgen: Honoraria, Research Funding, Speakers Bureau; Abbvie: Research Funding; Seattle Genetics: Research Funding; Jazz: Honoraria. Cortes:Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Arog: Research Funding; Astellas Pharma: Consultancy, Research Funding; Novartis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.