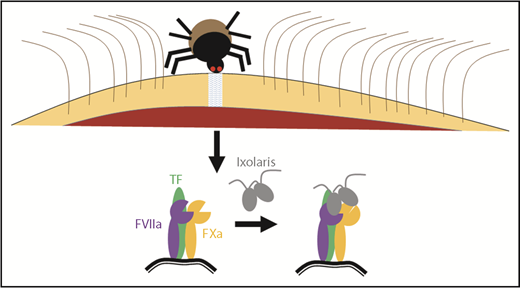
If you want to identify novel anticoagulant mechanisms, you should ask the experts: hemovores. In this issue of Blood, De Paula and colleagues delineate the unique mechanism by which the tick salivary anticoagulant Ixolaris inhibits the initiation of clotting, by blocking the tissue factor (TF)/factor VIIa (FVIIa) complex from activating factor X (FX) to FXa (see figure).1 Ixolaris is a Kunitz-type protease inhibitor, with similarity to human tissue factor pathway inhibitor (TFPI). TFPI inhibits TF/FVIIa through a 2-step mechanism.2 First, TF/FVIIa activates some FXa. Next, the first Kunitz domain (K1) and the second Kunitz domain (K2) of TFPI simultaneously bind the FVIIa and FXa active sites, respectively, forming the inhibitory TF/FVIIa/FXa/TFPI quaternary complex. In this way, TFPI allows some FXa generation before TF/FVIIa inhibition can be achieved. The tick Ixodes scapularis, the common deer tick that is a vector for Lyme disease, utilizes a variation of this mechanism to more completely prevent coagulation in its prey. Its salivary protein Ixolaris forms a similar inhibitory quaternary complex, but, unlike TFPI, does not require FX activation.3 Rather, Ixolaris binds either FX or FXa with similar affinity.
The saliva of hemovores, such as ticks, contains many anticoagulant molecules, which prevent the host from clotting so that the hemovore may feed. De Paula et al demonstrated that 1 tick anticoagulant, Ixolaris, works by binding the TF/FVIIa/FXa complex, similar to human TFPI. However, unlike TFPI, Ixolaris binds an exosite on FXa that induces a conformational change in its active site and loss of proteolytic function, a newly uncovered mechanism for regulating FXa function and limiting coagulation.
The saliva of hemovores, such as ticks, contains many anticoagulant molecules, which prevent the host from clotting so that the hemovore may feed. De Paula et al demonstrated that 1 tick anticoagulant, Ixolaris, works by binding the TF/FVIIa/FXa complex, similar to human TFPI. However, unlike TFPI, Ixolaris binds an exosite on FXa that induces a conformational change in its active site and loss of proteolytic function, a newly uncovered mechanism for regulating FXa function and limiting coagulation.
De Paula et al performed nuclear magnetic resonance (NMR) analyses to determine how Ixolaris is able to inhibit TF/FVIIa in the absence of FX activation. These studies revealed several interesting observations. First, Ixolaris binds to a heparin-binding exosite on FX/FXa, while leaving the FXa active site open. In this way, Ixolaris is similar to another hemovore anticoagulant, nematode anticoagulant protein c2 (NAPc2), with 1 important difference: NAPc2 is commonly used to study the activity of the TF/FVIIa/FXa complex, because it leaves FXa functional.4 By contrast, Ixolaris does not block the active site but does induce conformational changes that disrupt the orientation of the catalytic residues. Closer analysis revealed a specific loop in FXa that is involved in this allosteric regulatory mechanism.
Second, the interaction between Ixolaris and FX/FXa requires both the K1 and the K2 domains of Ixolaris. In this way, Ixolaris is similar to human TFPI. Removal of the K1 domain from TFPI, such as through cleavage by neutrophil elastase, results in a substantial loss of inhibitory activity, even though the K2 domain is responsible for binding the FXa active site.5 This observation suggested interactions between K1 and an exosite on FXa. De Paula et al were able to show that the Ixolaris K1 domain binds the FXa heparin-binding exosite through a series of salt bridges. It is possible that TFPI functions in a similar manner and that the structure of Ixolaris may guide future TFPI mutagenesis studies.
Third, the K1 and K2 domains of Ixolaris are adjacent to each other, rather than spaced. TFPI is often depicted using a beads-on-a-string model, in which the beads are the Kunitz domains.2 In this model, the Kunitz domains are independent and very flexible, relative to each other. The linker regions between the Kunitz domains of TFPI are predicted to be unstructured themselves, and so computer-generated models of the full-length protein have supported this concept.6 The present study by De Paula is the first to show the structure of a multi-Kunitz domain, TFPI-like protein, and directly contradicts this model. Instead of being spaced apart, this work showed that K1 and K2 are closely associated, which makes sense as both of these domains need to be able to bind FXa. It is possible that the association between K1 and K2 is a result of the short linker region present in Ixolaris (4 amino acids) and may not translate to TFPI, which has a longer linker between these domains (21 amino acids). Alternatively, the linker region lengths of TFPI vary considerably across species, so it is also possible that they do not significantly influence the structure and function of the Kunitz domains. Shortening the linkers may thus allow for determination of the TFPI structure using a similar methodology to the one presented here.
The saliva of hemovores, such as ticks, flies, nematodes, and leeches, is filled with anticoagulant proteins that enable these species to feed. Some of these proteins have evolved to become especially potent inhibitors of coagulation enzymes. For example, leech hirudin inhibits thrombin with subpicomolar affinity.7 Others have revealed new points of regulation in the coagulation cascade. The tick anticoagulant TIX-5 was used to show the critical role of FXa-mediated factor V activation in the initiation of thrombin generation.8 Here, studies of Ixolaris revealed how exosites on FXa can control the folding and function of its protease domain. As we continue to search for novel anticoagulant therapies, we can look to our hemovore friends for guidance. After all, they are the experts.
Conflict-of-interest disclosure: The author declares no competing financial interests.