Introduction: Loss of self-renewal of hematopoietic stem cells (HSC) is a major roadblock to cell engineering therapies. Small molecules have been identified that promote HSC expansion. We recently identified UM171, StemReginin1 (SR1) and valproic acid (VPA) as strongest agonist for expansion of cord blood (CB) CD34+CD45RA- and CD34+CD45RA-EpcrHigh (EpcrHg) HSC-enriched cells of 12 molecules tested. In addition, we identified a novel putative stem cell agonist in L-Ascorbic acid 2-phosphate (AA2P), a derivative of vitamin C. Using response surface methodology and machine learning, we identified a series of Stem Cell Agonist Cocktails (SCAC) composed of these 4 agonists at varying concentrations. The objectives of this study were to characterize the in vitro properties of AA2P and SCACs on CB HSC and, test the capacity of AA2P- and SCAC-expanded CB CD34+ cells to support hematopoietic recovery and long-term (LT) engraftment after transplantation.
Methods: Predictive models for HSC expansion (CD34+CD45RA- and EpcrHg) promoted by SR1, UM171, VPA, and AA2P were built by design of experiments. The data was then used to train a neural network. These were used as predictive tools to derive a series of SCAC composed of different concentrations of the 4 agonists (Table 1). CB expanded HSPC were characterized after 14 days of culture. Migration of HSPCs toward SDF-1 was tested in a transwell assay. Serial and limit dilution transplant assays in NSG mice were done to characterize the capacity of SCAC to support expansions of short-term (ST) and LT HSC.
Results: First, we investigated the capacity of AA2P to act as an HSC agonist. AA2P was unable on its own to expand EpcrHg cells but promoted cell growth and the expansion of CD34+CD45RA- HSPC (2-fold, p<0.05), a property shared by L-Ascorbic acid. Moreover, AA2P-expanded HSPCs enhanced ST platelet engraftment when compared to serum-free medium (SFM) control (p=0.053, n=3).
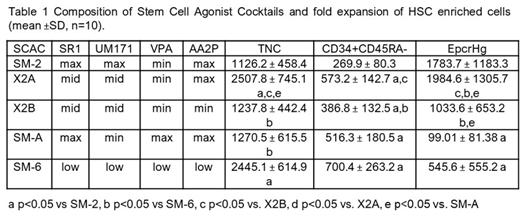
Next, we tested the activity of SCACs presented in Table 1. Varying the concentrations of the small molecules profoundly impacted cell growth and the type of HSPC expanded (Table 1). For instance, SM-2 with high UM171 provided high expansion of EpcrHg, but low level of overall cell growth. SM-A and SM-6 maximized expansion of CD34+CD45RA- cells but had lower expansion of EpcrHg due partly to lower UM171. X2A was unique as it produced balanced expansion of EpcrHg and CD34+CD45RA- cells. Lowering AA2P concentration in X2A significantly reduced the expansion of both HSC-enriched fractions (X2B, Table 1). Moreover, most SCACs enhanced expansion of HSC-enriched cells (CD34+CD45RA-CD38-CD90+CD133+, p<0.05 vs SFM) and that of lymphoid-primed multi-potential progenitors and multipotent progenitors vs. SFM cultures (p<0.05), but not of downstream progenitors.
Since homing to the bone marrow (BM) is a key step towards engraftment, we investigated whether SCACs influenced the expression of homing receptors and the migration activity of HSPCs. SCAC expanded HSPCs were characterized with elevated fucosylation of PSGL-1 known to favor homing and engraftment (e.g. 82 ± 2% vs. 42 ± 8% for X2A vs. SFM CD34+ cells, p<0.01, n=4). Also, most SCAC-expanded CD34+ cells showed improved migration towards SDF-1 (e.g. 22 ± 6% vs. 13 ± 9% for X2A vs. SFM CD34+ cells, p<0.05, n=4).
The capacity of SCAC-expanded HSPCs to support engraftment is still ongoing. Current results showed that X2A-expanded HSPCs provided the strongest ST recovery of platelets and leucocytes of all SCACs-HSPC, superior also to that seen with UM171-expanded HSPCs and non-cultured HSPCs (p<0.05, n=2-3). Further, LT human BM reconstitution was notably better for X2A- and SM-6-expanded HSPCs than other SCAC-expanded cells (p<0.05 vs SM2, n=2). Moreover, reducing AA2P in X2A resulted in a loss in ST and LT engraftment activity (p<0.05, n=2). Secondary transplants and limit dilution assays are ongoing to provide further insights into the impact of SCACs on the production and self-renewal activity of HSCs.
Conclusion: Our study reveals that AA2P promotes cell growth and can synergize with strong stem cell agonists to promote the expansion of ST and LT engrafting HSPCs. The engraftment properties of SCAC-expanded HSPCs was highly dependent on the concentrations of the small molecules due in part to negative interactions amongst some of the agonists. Gene expression studies are ongoing to define the transcriptional landscape of HSPC produced with these SCACs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.