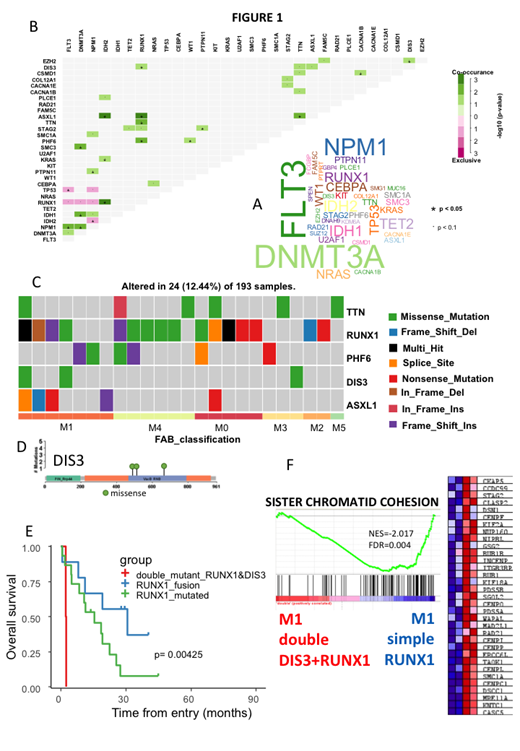
Defective in Chromatid Joining (DIS3) is a highly conserved exoribonuclease, involved in the 3'-5' degradation of RNAs, which has a major role as a post-transcriptional checkpoint in eukaryotic cells. The controlled degradation of unnecessary RNA transcripts from the cell by the DIS3 family enzymes has been shown to be essential for cell division and mitosis. Exome- sequencing analyses have demonstrated the presence of DIS3 mutations in several cancers with high incidence in patients with multiple myeloma but its role in acute myeloid leukemia (AML) has not been studied. AML is a malignancy characterized by clonal expansion and differentiation arrest of myeloid progenitors in the bone marrow and peripheral blood. M0 and M1 FAB subtypes are both poorly differentiated AML and RUNX1 mutations are associated with poorer outcome. In this work we asked whether we can confirm in the TCGA AML cohort, that RUNX1 mutations were associated with a prognostic significance and whether other co-existing mutations can influence the overall survival (OS). In this cohort of 193 patients, we have found that the presence of RUNX1 mutation is present in patients with worst OS (log rank p-value=0.036, median OS 227 versus 335 months). We then asked if the association with other mutations has an impact on clinical point of view. With a minimum 3 mutations per gene present in the cohort (Fig.1A), RUNX1 was found to be significantly co-associated with mutations present in 4 other genes: PHF6, TTN, ASXL1 and DIS3. RUNX1 associated mutations affecting ASXL1, TTN and PHF6 did not appear to modify prognosis. However, patients presenting both RUNX1 and DIS3 mutations had a dramatically reduced overall survival (log rank p-value=6E-4, median OS 61 versus 334 months) (Fig.1B) suggesting a dramatic co-association of these two mutations independently of TTN, PHF6, and ASXL1. The two patients presenting a co-association of RUNX1 and DIS3 mutations were found to have a leukemia of M1 subtype. One patient with AML-M3 presented an isolated DIS3 mutation without co-association with a RUNX1 mutation (Fig.1C). DIS3 missense mutations found in the cohort affected VacB RNB protein domain, which is implicated in ribonuclease activity and RNA binding of the molecule (Fig.1D). Removing M3 subtype from the TCGA cohort, RUNX1 fusions were found to be associated with M2 subtype, RUNX1 mutations with M0 & M4 subtypes, and double mutant RUNX1/DIS3 in essentially in M1subgroup (p-value = 0.006976). Without M3, patients with double RUNX1/DIS3 mutations were found to exhibit the worst prognosis respectively as compared to patients presenting mutations or fusion of RUNX1 alone (log rank p-value=0.0042). We then compared the transcriptome of M1 patients with double DIS3-RUNX1 mutations as compared to M1 patients with simple RUNX1 mutations. As can be seen in Fig.1F, the first group harbored an important down-regulation of genes involved in the cohesin complex such as SMC1A, RAD21, STAG2, suggesting a major reduction of sister chromatid cohesion functionality (Normalized enrichment score = -2.017, FDR q-value=0.004). There was also a down-regulation of molecules implicated in DNA double strand repair such as MRE11A, RAD21, as well as that of MAD2L1 (a mitosis checkpoint) and centromere proteins such as CENPF. These results suggest that DIS3 mutations are associated with an important loss of control for the entry in mitosis.
Overall, we show here for the first time to our knowledge, that the occurrence of DIS3 mutation in AML-M1 patients presenting a RUNX1 mutation confers a dismal prognosis in terms of overall survival. The transcriptome of these patients harbors a loss of functionality for sister chromatid cohesion. The search for DIS3 mutations is therefore warranted in all AML at diagnosis and could be of decisional value for therapeutic strategies.
Turhan:Incyte: Consultancy, Honoraria; novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.