Background
The development of chimeric antigen receptor (CAR) T cell therapy has shown tremendous success in the past decade. Since the first two autologous CD19-targeting CAR-T treatments, Kymriah and Yescarta, were approved by the FDA, the research activities in this field are being significantly increased. However, the scientific and clinical efforts have been extensively focused on developing novel CAR-T treatments with better tumour control or reduced on-target off tumor cytotoxicity. The importance of CAR-T manufacturing optimization has been relatively underestimated. It has been reported by FDA that Kymriah has about 9 % of manufacturing failure rate. This failure could directly affect the survival of some patients. Accordingly, some of the patients were forced to received out-of-spec product, because of the limited time window for another manufacturing attempt. The goal of this study is to examine if the qualities and consistency of the CAR-T products could be improved by an automatic manufacturing process.
Methods
Major CAR-T manufacturing variabilities resulted from the manual processing steps. To address the issues, we have adapted CliniMACS Prodigy (Miltenyi Biotec, Bergisch Gladbach, Germany), an all-in-one, automatic and closed system for cell processing, for our CAR-T manufacturing. In this study, we have compared the differences of CAR-T product from manual and automated processes, including the cell viability, transduction efficiency, and cell subset distribution. The in vitro cytotoxic effects of CAR-T cells made manually and automatically were also conducted by incubating the CAR-T cells with Raji cells at effector to target ratio (E:T) from 3:1 to 1:3. In order to test the inter-Prodigy variability, we applied the PBMC sample from one individual patient into two devices, and compared the quality indexes for the final products. Finally, we have performed a POC (proof of concept) CD19-CAR-T trials, and 3 patients who received CD19-CAR-T cells manufactured by CliniMACS Prodigy were evaluated.
Results
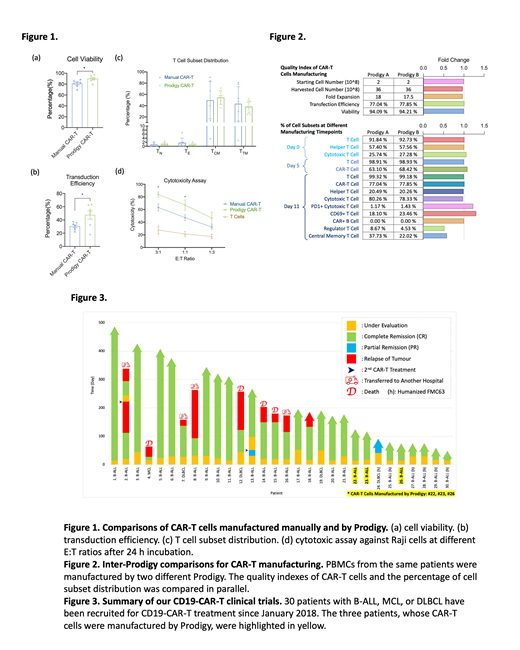
The comparisons between CAR-T cells from two manufacturing processes (n=6) suggested that the CAR-T cells made by Prodigy had significantly greater cell viability (Fig. 1a), higher transduction efficiency (Fig. 1b), and lower variations of cell subset distribution (Fig. 1c). It also showed that the Prodigy-made CAR-T cells had statistically higher in vitro cytotoxicity against Raji cells (Fig. 1d), possibly benefited from its higher transduction efficiency of CD19-CAR. The result of inter-Prodigy comparisons (Fig. 2), including final quality control indexes and the percentage of cell subset of the intermediate and final products, were almost identical. Since January 2018, 30 patients (one MCL, four DLBCL, and 25 B-ALL) have been treated with our FMC63-based or humanized FMC63-based CD19-CAR-T cells (Fig. 3). Among these patients, 28/30 (93.3%) cases achieved MRD-negative completed remission (CR), and two cases reached partial remission (PR) after receiving CAR-T infusion. All of the three patients who received automated-manufactured CAR-T cells (Fig 3, highlighted in yellow) have achieved CR in less than 20 days and have no sign of relapse until last follow-up date.
Conclusions
Compared to traditional small molecule drugs or biologics, the manufacturing successful rate for autologous CAR-T cells is much more critical because each failure could be live-threatening. Our comparison studies have proved that the automated process exhibited improved qualities and consistencies of CAR-T products. Moreover, automatic process will not only increase the manufacturing stability, but significantly reduce the dependency on qualified technicians or scientists, which will greatly prompt the commercialization of CAR-T cell products.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.