Background
Graft versus host disease (GVHD) is the principal cause of non-relapse mortality after allogeneic hematopoietic stem cell transplantation (allo HCT) and is associated with intestinal microbial dysbiosis. Administration of microbial metabolite butyrate or microbial cocktails of butyrogenic bacteria reduce the severity of acute GVHD in mice. Furthermore, this intestinal microbiome-metabolome axis can be manipulated via dietary intervention in healthy humans with supplementation by defined quantities of resistant potato starch (RPS), an indigestible carbohydrate that is metabolized by intestinal anaerobic commensal bacteria to produce butyrate1. Hereon we aimed to study the feasibility and tolerability of RPS in allo HCT recipients to test the hypothesis that the patients' intestinal microbiome and butyrate levels could be rationally modified by administration of defined quantities of RPS.
Methods
Between May 8, 2017 and September 30, 2018, we performed a single-center prospective, single arm, pilot study. We recruited adults who were undergoing human leukocyte antigen-matched, related-donor myeloablative allo HCT. Participants received RPS (Bob's Red Mill®) 20 g package orally, once/day for the first 3 days followed by twice daily, from day -7 through day 100 after allo HCT. Feasibility was defined as adherence to ≥ 70 % of scheduled doses in ≥ 60 % of patients. The primary objective was to test adherence to scheduled doses of RPS. Secondary endpoints included assessing tolerability of RPS and its ability to alter representation of RPS-degrading and butyrate-producing bacteria as well as butyrate levels in the intestines of allo HCT recipients. Stool samples were collected in the OMNIgene-Gut® (DNA Genotek) collection kit at baseline before intake of RPS, at time of nadir (day 7-10), engraftment (day 12-18), at day 100, and additional samples were also collected. Fecal microbiome was determined by 16S rRNA gene sequencing and butyrate by liquid chromatography.
Results
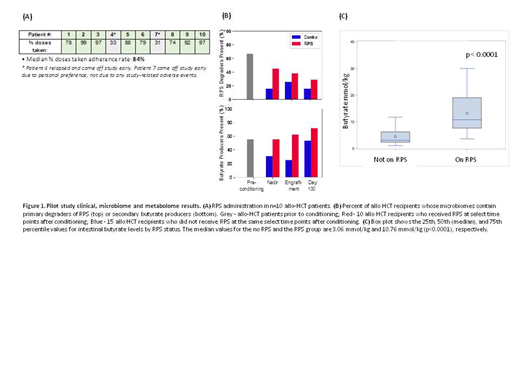
Ten subjects were enrolled. The median age was 57 years (range 52-62 years). All subjects received GVHD prophylaxis with tacrolimus and methotrexate as well as antibiotic prophylaxis with levaquin, and were treated for neutropenic fever with IV cefepime (90%) or IV vancomycin along with IV aztreonam (10%). One patient developed biopsy proven stage I acute GI GVHD with overall grade II acute GVHD (10%). Feasibility exceeded the preset goal of ≥ 70% adherence to scheduled dosages in ≥ 60 % of patients as 8 of the 10 patients (80%) received ≥ 70 % of scheduled doses (Figure 1A). No adverse effects/toxicities attributed to RPS were observed and longitudinal specimens were collected successfully. There was greater abundance of intestinal RPS-degraders such as Ruminococcus bromii, R. lactaris, R. gnavus, and Bifidobacterium spp and butyrate-producers such as Roseburia spp, Faecalibacterium prausnitzii, Eubacterium rectale, and Anaerostipes spp in the 10 patients receiving RPS compared to 15 historical controls after allo HCT (Figure 1B). Butyrate levels were significantly higher in the participants when they were on RPS as compared to pre RPS intake [median (interquartile range): 10.76 (7.62, 19.05) vs. 3.06 (2.32, 6.21) mmoL/kg, p<0.0001, respectively] (Figure 1C).
Conclusions
Our pilot study of dietary RPS demonstrated feasibility and safety and showed that RPS administration increased butyrate-producing bacteria with a concomitant increase in butyrate levels. A Phase II clinical trial built on these observations to test the efficacy of RPS in mitigating acute GVHD by altering the intestinal microbiome and its metabolites is underway (www.clinicaltrials.gov: NCT02763033).
1. Venkataraman A, Sieber JR, Schmidt AW, Waldron C, Theis KR, Schmidt TM. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome. 2016;4(1):33.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.