Background: The combination of all-trans-retinoic acid (ATRA) and arsenic trioxide (ATO) is superior to ATRA plus chemotherapy in the treatment of standard risk patients (pts) with newly diagnosed APL. MRD monitoring has been successfully utilized for the early identification of relapse. Qualitative PCR has been superseded with the more accurate real-time quantitative PCR (RQ-PCR) for MRD detection in APL.
Methods: We reviewed pts with newly diagnosed APL treated at our institution on 3 consecutive prospective clinical trials, using the combination of ATRA and ATO, with or without gemtuzumab ozogamicin (GO). GO was given to High risk pts (WBC >10 × 109/L) and pts with rising WBC. Real-time quantitative RT-PCR (RQ-PCR) was used to measure PML-RARα in bone marrow (BM) and peripheral blood (PB) specimens. We sought to determine the value of MRD monitoring in patients with APL treated with this regimen.
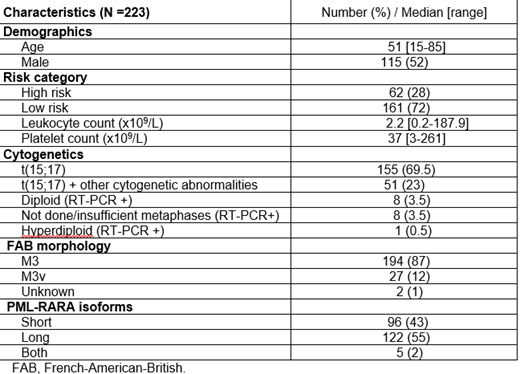
Results: A total of 223 pts with APL have been followed from July 2002 to March 2019 with a total of 2007 samples (1622 BM, 385 PB) analyzed with a median number of samples of 8 per pt (range, 1-43). Median follow up is 55.6 months (range, 1-198). MRD positivity decreased over time on therapy; 218 pts (98%) were MRD positive after induction, while only 2 pts (1%) were positive after the first cycle of consolidation. Eight pts (3.5%) had positive MRD (all ≤0.1) during consolidation or after completing treatment but became negative after repeated MRD testing and none of them relapsed. Overall, seven pts relapsed (5 with high risk disease and 2 with low risk) and The median time to relapse after achieving CR was 9.4 months (range, 7.9-79.5).The time to the first relapse was between 7.9-12.4 months except for the pt who relapsed after 79.5 months (low risk pt), Among the high risk pts, molecular relapse preceded hematological relapse by 3.7 weeks (range, 2.1-4.1). There was a correlation between quantitative PCR values on PB and BM samples obtained concomitantly (r2=0.67, p=0.048).
Conclusions: MRD monitoring may be useful for early detection of relapse in pts with high risk APL within first year after completion of therapy. Late molecular relapse is very rare and does not justify universal monitoring especially in standard risk patients. These data support the lack of need for MRD monitoring after completion of consolidation in pts with standard risk APL treated with ATRA plus ATO.
Kantarjian:Novartis: Research Funding; Takeda: Honoraria; Agios: Honoraria, Research Funding; Ariad: Research Funding; Daiichi-Sankyo: Research Funding; Cyclacel: Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Research Funding; Immunogen: Research Funding; BMS: Research Funding; Astex: Research Funding; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Jazz Pharma: Research Funding. Kadia:Celgene: Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Bioline RX: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees. Garcia-Manero:Merck: Research Funding; Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding. Jabbour:BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Takeda: Consultancy, Research Funding. Borthakur:Incyte: Research Funding; Merck: Research Funding; Strategia Therapeutics: Research Funding; Janssen: Research Funding; GSK: Research Funding; Agensys: Research Funding; Oncoceutics, Inc.: Research Funding; Argenx: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; BioTheryX: Membership on an entity's Board of Directors or advisory committees; AbbVie: Research Funding; Eli Lilly and Co.: Research Funding; BMS: Research Funding; Polaris: Research Funding; NKarta: Consultancy; FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Xbiotech USA: Research Funding; Arvinas: Research Funding; PTC Therapeutics: Consultancy; Cantargia AB: Research Funding; Tetralogic Pharmaceuticals: Research Funding; Eisai: Research Funding; AstraZeneca: Research Funding; Cyclacel: Research Funding; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer Healthcare AG: Research Funding; Oncoceutics: Research Funding. Short:Takeda Oncology: Consultancy, Research Funding; AstraZeneca: Consultancy; Amgen: Honoraria. Alvarado:Jazz Pharmaceuticals: Research Funding; Abbott: Honoraria. Daver:Karyopharm: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Servier: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Astellas: Consultancy; BMS: Consultancy, Research Funding; Immunogen: Consultancy, Research Funding; Forty-Seven: Consultancy; Agios: Consultancy; Hanmi Pharm Co., Ltd.: Research Funding; Celgene: Consultancy; Glycomimetics: Research Funding; Otsuka: Consultancy; NOHLA: Research Funding; Sunesis: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Jazz: Consultancy; Novartis: Consultancy, Research Funding. Cortes:Novartis: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; BiolineRx: Consultancy; Immunogen: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding; Biopath Holdings: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding. Ravandi:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Selvita: Research Funding; Xencor: Consultancy, Research Funding; Macrogenix: Consultancy, Research Funding; Menarini Ricerche: Research Funding; Cyclacel LTD: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.