Introduction: Both lenalidomide and monoclonal antibodies targeting the immune checkpoint PD-1, including nivolumab, have single agent activity in subsets of patients with Hodgkin lymphoma (HL) and Non-Hodgkin lymphoma (NHL). Lenalidomide mediates increased T cell IL-2 production and may enhance T and NK cell response to immune checkpoint blockade resulting in synergistic activity, but with potential for overlapping immune related toxicities. We conducted a phase I study to determine the safety, tolerability, and maximum tolerated dose (MTD) of nivolumab and lenalidomide in patients with relapsed/ refractory (R/R) HL and NHL.
Methods: Patients (age ≥18 years) with R/R B-cell NHL who were transplant ineligible or patients with R/R HL with ≥ 2 prior lines of treatment were eligible (NCT03015896). Inclusion criteria included ECOG performance status ≤ 2, creatinine clearance ≥ 40 mL/min, platelets ≥ 100,000/mm3, absolute neutrophil count (ANC) ≥ 1,500/mm3, and bilirubin ≤ 1.5 times the upper limit of normal. The primary objective was to determine the MTD of lenalidomide when combined with nivolumab given at standard fixed dosing. Nivolumab was administered as 240 mg IV every 2 weeks; lenalidomide was administered orally on days 1-21 of 28-day cycles. Aspirin 81 mg daily was required for thromboprophylaxis in patients not already receiving therapeutic anticoagulation. Dose escalation of lenalidomide was performed using a 3+3 design, with dose levels (DL): 10 mg (DL -1), 15 mg (DL 1), and 20 mg (DL 2). Dose limiting toxicity (DLT) was defined within cycle 1 as grade Gr 3 or 4 non-hematologic toxicity, Gr 3/4 febrile neutropenia, any Gr 5 toxicity, or either ANC ≤ 500/mm3 or platelets < 25,000/mm3 persisting > seven days. Adverse events (AEs) were reported using CTCAE Version 4.0. Responses were investigator assessed according to the 2014 Lugano criteria.
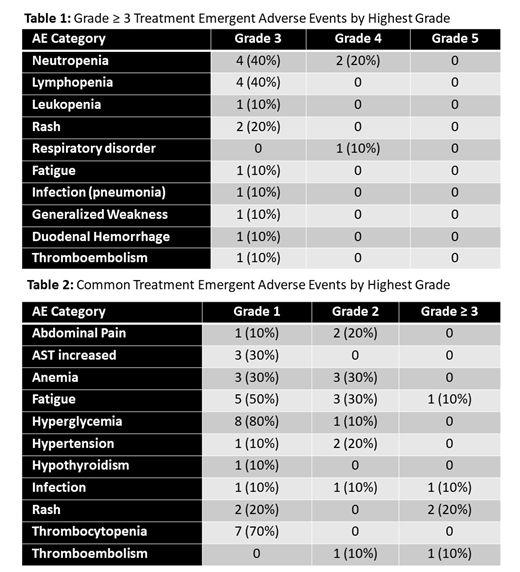
Results: Ten patients were enrolled. The median age was 68.5 (range 23-80), 5 were male, and the histologic diagnoses included diffuse large B cell lymphoma (DLBCL) in 5 patients (1 GCB, 4 non-GCB including 2 double expressor), high grade B cell lymphoma (HGBCL) in 1 patient, HL in 3 patients, and lymphoplasmacytic lymphoma in 1 patient. Two DLTs occurred at DL 1: Gr 3 rash requiring dose interruption and subsequent dose reduction in one patient and Gr 3 generalized weakness in a patient with rapidly progressive disease, considered possibly related to therapy. Six patients were treated at DL -1 with no DLTs. Gr ≥ 3 AEs occurred in 9/10 patients (Table 1). Neutropenia occurred in 7/10 patients: Gr 4 in two patients (both DL1), Gr 3 in four patients, and Gr 2 in one patient. Gr 3 rash occurred in two patients, and Gr 3 lung infection (pneumonia), Gr 3 fatigue, and Gr 3 duodenal hemorrhage (at site of DLBCL involvement) all occurred once respectively. Thromboembolic events occurred in two patients, one Gr 3 and one Gr 2. One Gr 4 non-hematologic AE occurred, Gr 4 respiratory disorder (COPD exacerbation) considered unrelated to treatment. One patient with rapidly progressive HGBCL prior to study entry died on study due to disease progression. Immune related AEs included Gr 1 hypothyroidism and Gr 1 fever in one patient. Common non-hematologic AEs of any grade are summarized in Table 2.
Therapy related AEs lead to treatment discontinuation in one patient (Gr 3 rash and Gr 3 neutropenia); three additional patients required dose reductions of lenalidomide due to therapy related AEs. As of July 1, 2019, one patient remains on treatment. Therapy was discontinued for disease progression in five patients, including one patient who died on study from progressive disease. Three patients discontinued treatment without progression in order to pursue definitive therapy (axicabtagene ciloleucel, autologous transplant, and allogeneic transplant respectively). Three patients achieved an objective response, one complete response and two partial responses, with an additional three patients with best response of stable disease, two of whom discontinued for alternative therapies without progression of disease.
Conclusions: We determined the MTD in R/R HL and NHL to be 10 mg lenalidomide in combination with 240 mg nivolumab. Treatment discontinuation due to toxicity was uncommon as were immune related AEs. A pre-planned phase 2 study in DLBCL and dose expansion study in HL is underway to evaluate preliminary efficacy of this combination at the MTD.
William:Techspert: Consultancy; Celgene Corporation: Consultancy; Kyowa Kirin, Inc.: Consultancy; Guidepoint Global: Consultancy; Defined Health: Consultancy. Brammer:Celgene: Research Funding; Seatlle Genetics: Honoraria, Speakers Bureau. Christian:Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Immunomedics: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Triphase: Research Funding; Merck: Research Funding; Janssen: Research Funding; Cephalon: Research Funding; Bristol-Myers Squibb: Research Funding; Acerta: Research Funding; Celgene: Research Funding; Millennium Pharmaceuticals Inc: Research Funding. Maddocks:Celgene: Membership on an entity's Board of Directors or advisory committees; Teva: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; BMS: Research Funding; Merck: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.