Background: AlloHCT is a potentially curative treatment for patients with acute myeloid leukemia (AML) and other myeloid malignancies (MM), however has a 40% relapse rate. The 2-year post-relapse survival rate is less than 20%; sustainable remissions are rare. Multiple strategies to mitigate relapse have been employed with variable degrees of success. An as yet untested approach involves manipulation of the T-cell milieu in the post-alloHCT setting using CI. Preclinical animal models of tumors have shown that blockade of PD-1 by monoclonal antibodies(mAbs) can enhance the anti-tumor immune response and result in tumor rejection, suggesting that host mechanisms limit the antitumor response. A murine model of an anti-PD-1 mAb given at the time of transplant showed that PD-1/PD-L1 interactions decrease acute GVHD, but increase chronic GVHD suggesting that PD-1 pathway modulation may provide unique opportunities for stimulating immune regulation post-alloHCT. Use of ipilimumab (I) to treat post-transplant MM resulted in a complete response rate of 42% and decreased the Treg/Tconv cell ratio, consistent with enhancement of the graft-versus-tumor effect. The CPIT-001 Trial demonstrated the feasibility and safety of combined CI with I and nivolumab (N) as consolidation following autologous stem cell transplantation (ASCT) for high-risk hematological malignancies as well as a 67% PFS rate at 18 months post-ASCT, a significant improvement as compared with historical data.
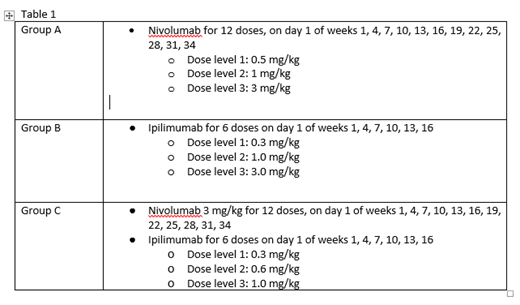
Study Design: The study employs a 3x3 design with intrapatient dose escalation. Patients will alternately be assigned to receive either N or I as a single agent. If the safety endpoint is met for each group, enrollment to the combination CI group will open. See table 1 for dosing. For all Groups, intrapatient dose escalation will be utilized. If the patient tolerates 28 days of treatment, he/she will be escalated to the next dose level and the next patient will be enrolled. Enrollment must occur within 60 days prior to HCT conditioning to allow for collection of baseline samples. Patients will start CI therapy 60 to 100 days after stem cell infusion when acute GvHD is adequately controlled on a prednisone dose of 20 mg daily or less, organ function, and peripheral counts are adequate. Key inclusion criteria at study start up included patient age 75 years or less with high risk AML or MDS undergoing alloHCT with a matched-related or 10/10 unrelated donor who were receiving non-myeloablative conditioning.
Bone marrow aspiration will be performed for response evaluation approximately every 3 months post CI initiation. The primary objective is to assess the safety of CI in this patient population. Secondary objectives include efficacy, assessment of blood immune reconstitution, phenotype and TCR repertoire by sequencing, assessment of tumor site immune phenotype, TCR repertoire and PD-L1/2 expression both prior to alloHCT conditioning and at relapse. The tertiary objective is to identify specific intestinal microbial strains associated with improved outcomes in alloHCT patients treated with CI. Microbial composition in stool samples of patients will be analyzed at screening, at engraftment, at various time points post-transplant, and at time of relapse, as it occurs.
Study Experience Thus Far: The study opened to accrual in May of 2017. Four patients have signed informed consent. Two patients have been treated with CI. One patient withdrew consent prior to treatment and a second patient is currently status post alloHCT, but has not yet reached day 60. One patient received 2 doses of N and the second patient received 1 dose of I.
Accrual to trial has been slower than anticipated. A detailed analysis of the patient screening log was completed. It was determined that 139 patients had been screened for trial. Major reasons for being ineligible included a diagnosis of ALL (34 patients; 24%), use of a haploidentical donor (30 patients; 22%), use of a full dose conditioning regimen (14 patients; 10%), diagnosis of CML (12 pateints; 10%), age greater than 75 (12 patients; 9%), diagnosis of CMML (12 patients; 9%). Following this analysis, an amendment was filed to include patients receiving haplo identical transplant and/or myeloablative conditioning, and to expand diagnosis to include all MM including CMML, CML blast crisis, and myelofibrosis.
Koprivnikar:Amgen: Speakers Bureau; Pfizer: Honoraria; Abbvie: Speakers Bureau; Novartis: Speakers Bureau. McCloskey:Jazz: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Takeda: Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Abbvie: Speakers Bureau; Novartis: Speakers Bureau. Rowley:Fate Therapeutics: Consultancy; Allergan: Equity Ownership.
Nivolumab - immunomodulation Ipilimumab - immunomodulation
Author notes
Asterisk with author names denotes non-ASH members.