Introduction
Axi-cel is approved in the US for the treatment of adult patients with relapsed or refractory large B cell lymphoma after 2 or more lines of systemic therapy. A post-marketing study is ongoing in the US utilizing the infrastructure created by the Center for International Blood and Marrow Transplant (CIBMTR) for post-approval safety and efficacy assessment and to follow these patients for 15 years through the established cellular therapy registry. This is the first year analysis of this study.
Methods
From October 18, 2017 to May 1, 2019, 453 axi-cel recipients were voluntarily reported to the CIBMTR. Of these, 295 patients from 43 US centers that have at least the first follow-up assessment submitted at 3 months were included in this analysis. Median follow-up was 6 months (range, 1-14 months).
Results
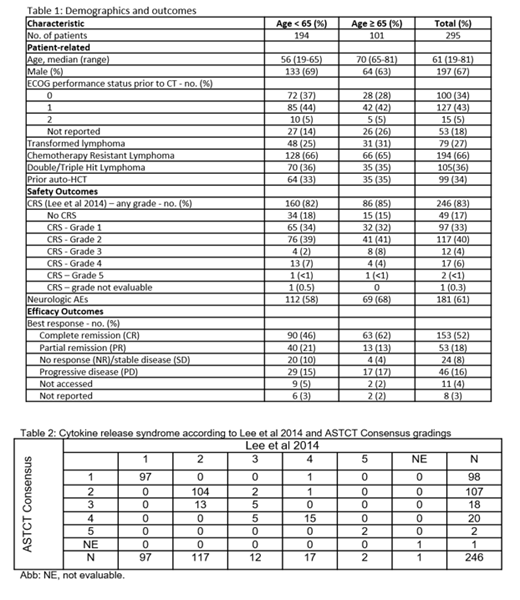
The median age overall was 61 years, 101 (34%) patients were ≥ 65 years, and 197 (67%) patients were male (Table 1). Baseline clinical characteristics included Eastern Cooperative Oncology Group (ECOG) performance score 0-1 (77%), transformed lymphoma (27%), double-hit lymphoma (36%), prior autologous transplant (34%), and chemotherapy-resistant disease (66%) prior to axi-cel. The median time from diagnosis to axi-cel infusion was 18 months (range 2-274 months). Overall response rate (ORR) was 70% (complete response [CR] 52% and partial response [PR] 18%). Patients ≥ 65 years were generally comparable vs younger patients with a slightly better CR rate (62% vs 46%, p=0.03) but similar overall response rate (CR+PR, 75% vs 67%, p=0.26). Cytokine release syndrome (CRS) of any grade was reported in 83% of patients. Incidence of Grades ≥ 3 CRS according to Lee et al 2014 was 11%, and was 14% according to American Society for Transplantation and Cellular Therapy (ASTCT) Consensus Grading (Table 2). Two patients died due to CRS. Median time to any grade CRS was 3 days (range, 1-17 days), and 94% of CRS cases resolved with a median duration of 7 days (range, 1-121 days). Among patients with CRS, tocilizumab, corticosteroids and siltuximab were used in 70%, 26% and 1% of cases, respectively. Neurologic adverse events (AEs) of any grade occurring after axi-cel infusion were reported in 181 (61%) patients. One patient was reported to die from cerebral edema. Additional information on neurologic AE severity will be presented. The median time to onset of any grade neurologic AEs was 6 days (range, 1-82 days), and 88% resolved by time of data submission with a median duration of 8 days (range, 1 to 105 days). Corticosteroids were used in 56% of patients for treatment. Patients ≥ 65 years had comparable CRS (85% vs 82%, p=0.62), grades ≥ 3 CRS (13% vs 9%, p=0.62), and neurologic AEs (68% vs 58%, p=0.13) vs patients < 65 years of age. Prolonged cytopenias (thrombocytopenia and neutropenia), as defined by an inability to recover within 30 days after the administration of axi-cel, occurred in 7% of patients. Preliminary data reveals 6 patients (2%) reported subsequent neoplasms: myelodysplasia (n=3), lung cancer (n=1), neuroendocrine tumor (n=1), and cutaneous squamous cell carcinoma (n=1); additional details, including pre-existing risk factors, will be addressed when the updated analysis is presented.
Conclusion
Post-approval axi-cel use reported in this registry study in the US, when compared to the registrational ZUMA-1 trial, includes a larger proportion of older patients, patients with transformed or double-hit lymphoma, and patients with a worse performance status. Despite these differences, best responses and toxicities are comparable to those reported for the ZUMA-1 trial. CRS severity assessment varied based on the grading method utilized, with a slightly higher rate of grade 3 CRS based on ASTCT Consensus Grading compared with Lee et al 2014. The safety and efficacy outcomes of patients ≥ 65 years at this early stage are comparable to those of younger patients, although further analysis with more follow-up is warranted.
Pasquini:Novartis: Research Funding; Kit Pharma: Research Funding; BMS: Research Funding; Pfizer: Other: Advisory Board; Amgen: Consultancy; Medigene: Consultancy. Locke:Cellular BioMedicine Group Inc.: Consultancy; Kite: Other: Scientific Advisor; Novartis: Other: Scientific Advisor. Herrera:Pharmacyclics: Research Funding; Kite Pharma: Consultancy, Research Funding; Immune Design: Research Funding; Merck: Consultancy, Research Funding; Genentech, Inc.: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; AstraZeneca: Research Funding; Adaptive Biotechnologies: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Gilead Sciences: Consultancy, Research Funding. Siddiqi:Seattle Genetics: Speakers Bureau; BeiGene: Research Funding; Celgene: Research Funding; TG Therapeutics: Research Funding; Kite: Research Funding; Astra Zeneca: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie company: Consultancy, Research Funding, Speakers Bureau; Juno: Consultancy, Research Funding; Janssen: Speakers Bureau. Ghobadi:Celgene: Consultancy; Wugen: Consultancy; Kite Pharma a Gilead Company: Consultancy, Research Funding, Speakers Bureau; EUSA: Consultancy. Dong:Gilead Inc: Other: Own Stock; Kite Pharma: Employment. Nikiforow:Kite/Gilead: Honoraria; Novartis: Honoraria; NKarta: Honoraria. Purdum:Kite Pharma: Employment. Horowitz:Sanofi: Other: Unrestricted educational and research grant, Research Funding; Chimerix: Other: Unrestricted educational and research grant; CSL Behring: Other: Unrestricted educational and research grant, Research Funding; Regeneron: Other: Unrestricted educational and research grant; Kite Pharma/Gilead: Other: Unrestricted educational and research grant, Research Funding; Daiichi Sankyo: Other: Unrestricted educational and research grant; GlaxoSmithKline: Other: Unrestricted educational and research grant; Janssen: Other: Unrestricted educational and research grant, Research Funding; Gamida Cell: Other: Unrestricted educational and research grant, Research Funding; Actinium: Other: Unrestricted educational and research grant; Amgen: Other: Unrestricted educational and research grant; Bristol-Myers Squibb: Other: Unrestricted educational and research grant, Research Funding; Magenta: Consultancy, Other: Unrestricted educational and research grant; Mesoblast: Other: Unrestricted educational and research grant, Research Funding; Miltenyi Biotech: Other: Unrestricted educational and research grant, Research Funding; Oncoimmune: Other: Unrestricted educational and research grant; Pharmacyclics: Other: Unrestricted educational and research grant; Seattle Genetics: Other: Unrestricted educational and research grant; Shire: Other: Unrestricted educational and research grant. Hooper:Kite Pharma Inc: Employment, Other: Owner Stock. Kawashima:Kite: Employment. Jacobson:Bayer: Consultancy, Other: Travel Expenses; Novartis: Consultancy, Honoraria, Other: Travel Expenses; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Precision Biosciences: Consultancy, Other: Travel Expenses; Pfizer: Consultancy, Research Funding; Humanigen: Consultancy, Other: Travel Expenses; Celgene: Consultancy, Other: Travel Expenses.
Author notes
Asterisk with author names denotes non-ASH members.