Introduction: The use of lenalidomide (LEN) and bortezomib (BTZ) in newly diagnosed multiple myeloma (MM) patients (pts), along with continuous or maintenance therapy paradigm have improved survival outcomes. However, many pts progress while on these agents or discontinue them due to toxicity. There is a need for novel, efficacious, and tolerable regimens that can treat MM pts who are exposed or refractory to LEN or BTZ. The proteasome inhibitor carfilzomib and the anti-CD38 monoclonal antibody daratumumab have both been approved as single agents or as components of combination regimens for the treatment of RRMM. The combination of carfilzomib, dexamethasone, and daratumumab has been shown to be efficacious and safe in RRMM in the phase 1 study MMY1001 (Chari, Blood 2019). We present results from the primary analysis of CANDOR, a multicenter, phase 3, randomized study comparing carfilzomib, dexamethasone, and daratumumab (KdD) vs carfilzomib and dexamethasone (Kd) in RRMM pts.
Methods: RRMM pts with measurable disease who had received 1-3 prior lines of therapy, with partial response or better to ≥1 line of therapy were eligible. Pts were randomized 2:1 to KdD or Kd. All pts received carfilzomib (K) as a 30-min intravenous (IV) infusion on days 1, 2, 8, 9, 15, and 16 of each 28-day cycle (20 mg/m2 on days 1 and 2 during cycle 1 and 56 mg/m2 thereafter). Daratumumab (8 mg/kg) was administered IV on days 1 and 2 of cycle 1 and at 16 mg/kg once weekly for the remaining doses of the first 2 cycles, then every 2 wks for 4 cycles (cycles 3 to 6), and every 4 wks thereafter. All pts received 40 mg dexamethasone oral or IV weekly (20 mg for pts >75 years). The primary endpoint was progression-free survival (PFS). Secondary endpoints were overall response rate (ORR), minimal residual disease (MRD) negative-complete response at 12 months (threshold, 10-5 cells), overall survival (OS), time to response, and safety.
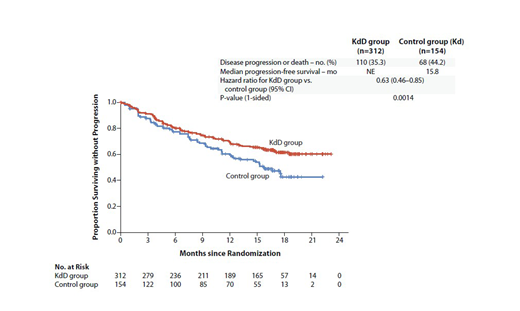
Results: 466 pts (KdD: 312; Kd: 154) from 102 sites worldwide were randomized. Baseline characteristics were balanced between the two arms. Median age was 64 years. Of the randomized pts, 42.3% and 90.3% received previous LEN- and BTZ-containing regimens, respectively. 33% of pts were LEN-refractory. The primary endpoint of PFS was met after a median follow-up of 16.9 mo and 16.3 mo for the KdD and Kd arms, respectively. Median PFS was not reached for the KdD arm vs 15.8 mo for the Kd arm (HR, 0.63; 95% CI, 0.46-0.85; P=0.0014; Figure). PFS HRs favored KdD vs Kd across prespecified subgroups. Importantly, median PFS (KdD vs Kd) was not reached vs 12.1 mo in the LEN-exposed group (HR, 0.52; 95% CI, 0.34-0.80), and was not reached vs 11.1 mo in the LEN-refractory group (HR, 0.45; 95% CI, 0.28-0.74). Median time to first response was 1 mo in the KdD and Kd arms. ORR was 84.3% vs 74.7% (P=0.0040), and the rate of complete response or better was 28.5% vs 10.4%. MRD-negative complete response rate at 12 mo was 12.5% for KdD vs 1.3% for Kd (P<0.0001). Median OS was not reached in either arm at a median follow-up time of 17 mo (HR, 0.75; 95% CI, 0.49-1.13; P=0.08). Median treatment duration was longer in the KdD than Kd arm (70.1 vs 40.3 wks). The incidence of grade ≥3 AEs was 82.1% and 73.9% in the KdD and Kd arms, respectively. Serious AEs occurred in 56.2% (KdD) and 45.8% (Kd). The rate of treatment discontinuation due to AEs was similar in both arms (KdD, 22.4%; Kd, 24.8%). The frequency of grade ≥3 cardiac failure was 3.9% (KdD) and 8.5% (Kd); rate of cardiac failure event leading to K discontinuation was similar in the arms (3.9% and 4.6%). 5 deaths were reported as treatment-related, all in the KdD arm (pneumonia, sepsis, septic shock, acinetobacter infection, and cardio-respiratory arrest [n=1 each]). Additional efficacy endpoints, including key subgroup analyses will be presented.
Conclusion: KdD resulted in a significant PFS benefit over Kd, with a 37% reduction in the risk of progression or death. Pts treated with KdD achieved deeper responses, with a nearly 10-times higher MRD negative-complete response rate vs Kd-treated pts. The PFS benefit of KdD was maintained across prespecified clinically important subgroups, particularly among LEN-exposed and LEN-refractory pts. AEs were generally manageable and the incidence of AEs leading to treatment discontinuation was similar in the arms. Overall, KdD was associated with a favorable benefit-risk profile and represents an efficacious new regimen for RRMM, including for LEN-exposed and/or LEN-refractory pts.
Usmani:AbbVie: Other: Personal fees; SkylineDx: Consultancy, Other: Grant, Personal fees, Research Funding; GSK: Consultancy, Research Funding; Seattle Genetics: Consultancy, Other: Grant, Personal fees, Research Funding; MundiPharma: Honoraria, Other: Personal fees; Takeda: Consultancy, Other: Grant, Personal fees, Research Funding, Speakers Bureau; Sanofi: Other: Grant, Personal fees, Research Funding, Speakers Bureau; Pharmacyclics: Other: Grant, Research Funding; Merck: Consultancy, Research Funding; Janssen: Consultancy, Other: Grant, Personal fees, Research Funding, Speakers Bureau; Celgene: Consultancy, Other: Grant, Personal fees, Research Funding, Speakers Bureau; BMS: Consultancy, Other: Grant, Research Funding; Array Biopharma: Grant, Personal fees, Research Funding; Amgen: Consultancy, Other: Grant, Personal fees, Research Funding, Speakers Bureau. Quach:Celgene Corp: Membership on an entity's Board of Directors or advisory committees, Other: investigator initiated clinical study; Grant, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: investigator initiated clinical study; Grant, Research Funding; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Other: Free drug for investigator-initiated study, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees. Mateos:Glicomimetics: Other: Personal fees; Pharmamar: Membership on an entity's Board of Directors or advisory committees; EDO: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees; Adaptive: Honoraria; GSK: Membership on an entity's Board of Directors or advisory committees, Other: Personal fees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: Personal fees. Landgren:Takeda: Other: Grants, Independent Data Monitoring Committee (IDMC); Rising Tides Foundation: Other: Grants; Amgen: Other: Grant, Personal fees; Seattle Genetics: Other: Grants; NIH: Other: Grants; FDA: Other: Personal fees; Glenmark: Other: Grants; MMRF: Other: Independent Data Monitoring Committee (IDMC); IMF: Other: Grants; LLS: Other: Grants; Perelman Family Foundation: Other: Grants; Karyopharm: Other: Grants; Adaptive: Other: Personal fees; Binding Site: Other: Personal fees; Celgene: Other: Grant, Personal fees; BMS: Other: Personal fees; Cellectis: Other: Personal fees; Juno: Other: Personal fees; Pfizer: Other: Personal fees; Merck: Other: Independent Data Monitoring Committee (IDMC); Janssen: Other: Grants, Personal fees, Independent Data Monitoring Committee (IDMC). Leleu:Sanofi: Honoraria; Takeda: Honoraria; Oncopeptide: Honoraria; Karyopharm: Honoraria; Amgen: Honoraria; Carsgen: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Janssen: Honoraria; BMS: Honoraria; Merck: Honoraria; GSK: Honoraria; AbbVie: Honoraria. Siegel:Novartis: Honoraria, Speakers Bureau; Karyopharm: Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Weisel:Janssen: Consultancy, Honoraria, Research Funding; Adaptive Biotech: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Juno: Consultancy; GSK: Honoraria. Yang:Amgen: Employment, Equity Ownership. Klippel:Amgen: Employment, Equity Ownership. Zahlten-Kumeli:Amgen: Employment, Equity Ownership. Dimopoulos:Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Sanofi Oncology: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Genesis Pharma: Research Funding; BMS: Consultancy; Celgene: Consultancy, Honoraria.
carfilzomib dexamethasone, and daratumumab triplet for relapsed or refractory multiple myeloma
Author notes
Asterisk with author names denotes non-ASH members.