Key Points
ST2, TNFR1, and REG3α, in post-HCT landmark analyses, associated with NRM in children ≤10 years and children/adults >10 years.
When pre-HCT biomarkers were included, ST2 remained significantly associated with NRM only in children ≤10 years.
Abstract
Assessment of prognostic biomarkers of nonrelapse mortality (NRM) after allogeneic hematopoietic cell transplantation (HCT) in the pediatric age group is lacking. To address this need, we conducted a prospective cohort study with 415 patients at 6 centers: 170 were children age 10 years or younger and 245 were patients older than age 10 years (both children and adults were accrued from 2013 to 2018). The following 4 plasma biomarkers were assessed pre-HCT and at days +7, +14, and +21 post-HCT: stimulation-2 (ST2), tumor necrosis factor receptor 1 (TNFR1), regenerating islet–derived protein 3α (REG3α), and interleukin-6 (IL-6). We performed landmark analyses for NRM, dichotomizing the cohort at age 10 years or younger and using each biomarker median as a cutoff for high- and low-risk groups. Post-HCT biomarker analysis showed that ST2 (>26 ng/mL), TNFR1 (>3441 pg/mL), and REG3α (>25 ng/mL) are associated with NRM in children age 10 years or younger (ST2: hazard ratio [HR], 9.13; 95% confidence interval [CI], 2.74-30.38; P = .0003; TNFR1: HR, 4.29; 95% CI, 1.48-12.48; P = .0073; REG3α: HR, 7.28; 95% CI, 2.05-25.93; P = .0022); and in children and adults older than age 10 years (ST2: HR, 2.60; 95% CI, 1.15-5.86; P = .021; TNFR1: HR, 2.09; 95% CI, 0.96-4.58; P = .06; and REG3α: HR, 2.57; 95% CI, 1.19-5.55; P = .016). When pre-HCT biomarkers were included, only ST2 remained significant in both cohorts. After adjustment for significant covariates (race/ethnicity, malignant disease, graft, and graft-versus-host-disease prophylaxis), ST2 remained associated with NRM only in recipients age 10 years or younger (HR, 4.82; 95% CI, 1.89-14.66; P = .0056). Assays of ST2, TNFR1, and REG3α in the first 3 weeks after HCT have prognostic value for NRM in both children and adults. The presence of ST2 before HCT is a prognostic biomarker for NRM in children age 10 years or younger allowing for additional stratification. This trial was registered at www.clinicaltrials.gov as #NCT02194439.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is the most effective cancer immunotherapy available to date. Although HCT can induce beneficial graft-versus-tumor effects and be potentially curative for a wide range of nonmalignant blood diseases, clinically significant acute graft-versus-host disease (aGVHD) continues to affect up to 50% of HCT recipients.1 The development of plasma aGVHD biomarkers2-6 has heightened interest in identifying measureable proteins that might provide meaningful information early in the course of HCT. Several studies in large cohorts,5,7,8 have reported prognostic aGVHD-related biomarkers that by definition identify the likelihood of a clinical event occurring in patients who have the medical condition of interest (HCT).9 Unfortunately, pediatric representation in these studies has been less than 10%, particularly for children age 10 years or younger. Assessment of pre-HCT biomarkers in large cohorts has also been lacking. This is particularly important, given the results of a retrospective Center for International Blood & Marrow Transplant Research (CIBMTR) study that identified risk factors for nonrelapse mortality (NRM) and survival among 1117 children with leukemia and chronic GVHD (cGVHD) who received a transplant either from related or from various unrelated donor sources. Factors associated with worse survival were age younger than 10 years, transplantation from HLA partially matched or mismatched unrelated donors, advanced disease at HCT, and Karnofsky/Lansky score <80.10 Age is a major risk factor that has raised speculation that informative aGVHD biomarkers might differ between adults and children.
To address this knowledge gap, we conducted a large prospective multicenter clinical trial to test aGVHD biomarkers in a predominantly pediatric cohort. On the basis of the CIBMTR study,10 we used the age of 10 years as a cutoff (age 10 years or younger and older than age 10 years) in our study. Four previously identified aGVHD plasma biomarkers that had been validated in largely adult study cohorts were assessed in the plasma pre-HCT (at day −7 before the preparative regimen) and at days +7, +14, and +21 post-HCT: stimulation-2 (ST2), tumor necrosis factor receptor 1 (TNFR1), regenerating islet–derived (REG3α), and interleukin-6 (IL-6). We first used ST2, TNFR1, REG3α, and IL-6 because they are the most validated biomarkers in adult cohorts and also in the ∼10% subset of pediatric patients who were included in a previous cohort.5 Therefore, we focused our analyses on these 4 biomarkers, and we used dynamic prediction of NRM by landmark analysis of competing risks first tested with post-HCT samples and then tested with pre-HCT samples. By using this landmark analysis, we found that testing post-HCT biomarkers during the first 3 weeks after HCT is significantly associated with NRM in children age 10 years or younger, and also in children and adults older than age 10 years, which confirmed findings in the pediatric population from previous adult studies that included children. In addition, we report for the first time that pre-HCT ST2 is significantly associated with NRM, particularly among children age 10 years or younger.
Patients and methods
Study population
The study accrued 415 HCT recipients between 2013 and 2018: 170 children age 10 years or younger and 245 patients older than age 10 years (including both children and adults). All patients were observed for at least 1 year. This study was approved by the respective institutional review boards at 6 adult and pediatric centers: Indiana University School of Medicine, Johns Hopkins University School of Medicine, Texas Children’s Hospital, Fred Hutchinson Cancer Research Center, Boston Children’s Hospital, and Children’s National Medical Center. Adult patients were solely from 2 centers. Informed consent was obtained from all patients or their legal guardians.
Sample preparation and ELISA
All plasma samples were prospectively collected and stored per institutional guidelines. Frozen samples were shipped to the S.P. laboratory at Indiana University for analysis. ST2, TNFR1, REG3α, and IL-6 were measured pre-HCT (day −7) and on days +7, +14, and +21 post-HCT as previously examined in the aGVHD setting.2-5,11-14 All of these biomarkers were measured by using sequential enzyme-linked immunosorbent assay (ELISA), as previously reported.2,4,11-14
Statistical analysis
Descriptive statistics of demographic variables and NRM, including frequencies and percentages, were summarized by age groups (age 10 years or younger vs older than age 10 years). χ2 or nonparametric Mann-Whitney U tests were performed to assess potential differences in demographic variables between age categories. Cumulative incidences of NRM and aGVHD were analyzed with relapse as a competing risk.15 The Aalen-Johansen estimator was used to nonparametrically estimate the cumulative incidence function (CIF) of NRM and aGVHD. To account for the time-dependent biomarker values (pre-HCT and at 7, 14, and 21 days post-HCT), we used landmark analyses.16,17 Separate CIFs were estimated by biomarker category (above and below the median at day 21 for the whole cohort, which is also a clinically relevant threshold based on previous studies)2,11-13 for each landmark time point. The biomarker value at this landmark time point was considered along with the patients who survived until that landmark. To statistically evaluate the difference of the CIFs of NRM and GVHD between the 2 biomarker groups, while accounting for the left truncation at each landmark point, we used 2-sample nonparametric linear tests.18 To perform a multivariable competing risks analysis, the biomarker values for all landmarks points were stacked into a single variable, and a semiparametric proportional cause-specific hazards model was fitted to the data.17 Hazard ratios (HRs) with 95% confidence intervals (CIs) were reported to demonstrate effect size. To account for the potential association across the repeated records for each patient, we used a proper sandwich variance estimator in a population-averaged estimation framework. For each model, the interaction between the biomarker and the landmark quantified the change of the association between each biomarker and the outcome over time. If the interaction was not statistically significant, then there was no evidence that the association between the biomarker and the outcome changed over time. The models also included an interaction term between the biomarker and patient’s age. A significant biomarker by age interaction indicated that the association between the biomarker and the outcome differed between age groups. Multivariable analyses were conducted using the following covariates: race/ethnicity, malignant disease, graft source, and aGVHD prophylaxis. A cause-specific analysis that included malignant disease type, disease risk index, and disease status at HCT was also performed in the subgroup of patients with malignant diseases. We sought to determine whether the combination of biomarkers improves the predictive accuracy for NRM. To compare the predictive accuracy across different baseline biomarkers, we used the time-dependent prediction error estimate proposed by Schoop et al.19 This measure estimates predictive accuracy in situations with time-to-event outcomes and competing risks. A lower value of the estimated prediction error indicates a better predictive performance. A value of P < .05 was considered significant.
Results
Patient demographics and outcomes by age group
The characteristics of the 415 patients accrued are provided in Table 1 and are dichotomized into 2 age groups; age 10 years or younger (41%) and older than age 10 years (59%); overall, the cohort included approximately 70% of pediatric patients defined as age 18 or younger. There was an overrepresentation of non-white/non-Hispanic (P = .0027), nonmalignant disease (P < .0001), lymphoblastic malignant disease (P = .05), marrow and umbilical cord grafts (P < .0001), and aGVHD prophylaxis with antithymocyte globulin, alemtuzumab, and posttransplantation cyclophosphamide (P < .0001) among the younger recipients compared with the older recipients.
NRM was our primary outcome of interest. We also investigated aGVHD grades 2 to 4 (n = 157), gut GVHD (n = 88) (because ST2 and REG3α have been associated with gut GVHD),5 and cGVHD (n = 105) as secondary outcomes. We were not able to investigate the association with severe aGVHD grades 3 to 4 (n = 43) and aGVHD-related deaths (n = 23) because of the small number of events.
Surprisingly, the cumulative incidence of NRM was not significantly different between patients age 10 years or younger, and those older than age 10 years (P = .41; Figure 1); NRM at 1 year was 10.8% vs 13%, respectively. Causes of mortality at 1 year are presented in supplemental Table 1 (available on the Blood Web site); alloreactivity (n = 31; 36%) and infection (n = 12; 14%) were the most common causes of NRM. Similarly, the cumulative incidence of aGVHD grades 2 to 4 at onset was also not significantly different between patients age 10 years or younger (37.2%) and those older than age 10 years (38.7%) (P = .52; supplemental Figure 1). For cGVHD, as expected, the cumulative incidence was higher in the older age group: 13% for those age 10 years or younger and 37% for those older than age 10 years (P = .0143; supplemental Figure 2).
Cumulative incidence curves of NRM by age group. Curves compare patients age 10 years or younger and older than age 10 years. The NRM at 1 year was 10.8% (95% CI, 5.52%-14.64%) for the age 0 to 10 years group and 13.0% (95% CI, 8.70%-17.26%) for the older than age 10 years group (P = .41).
Cumulative incidence curves of NRM by age group. Curves compare patients age 10 years or younger and older than age 10 years. The NRM at 1 year was 10.8% (95% CI, 5.52%-14.64%) for the age 0 to 10 years group and 13.0% (95% CI, 8.70%-17.26%) for the older than age 10 years group (P = .41).
Biomarkers in post-HCT samples and outcome by age
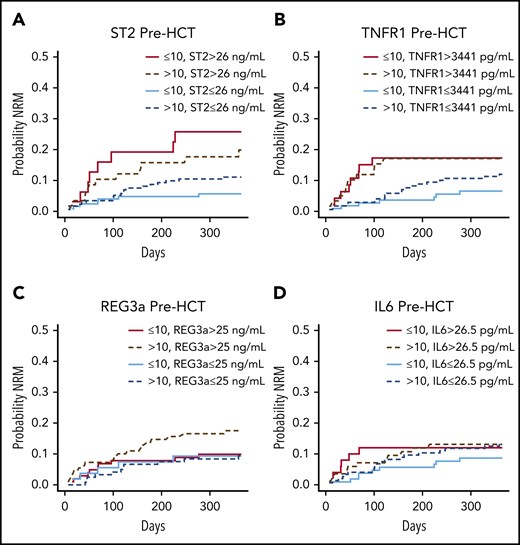
We first examined cumulative incidence curves of NRM by age group for the 4 biomarkers at days +7, +14, and +21 post-HCT. Low or high biomarker levels were defined by the median of the entire cohort at day +21 for each biomarker because it is similar to cutoffs used in previous studies.11-13 Medians and ranges of all biomarkers at all time points are presented in supplemental Table 2. Of note, biomarker values are consistently and significantly higher in the older group for pre-HCT measurements, consistent with a previous publication showing that age was associated with higher ST2 levels in the general population.20 ST2 was dichotomized at 26 ng/mL, TNFR1 at 3441 pg/mL, REG3α at 25 ng/mL, and IL-6 at 26.5 pg/mL. At day +21 post-HCT, there was a significant difference among children age 10 years or younger in the probability of NRM between high and low ST2 levels across time within 1 year (P = .002), whereas at earlier time points, differences were not statistically significant (Figure 2). High TNFR1 at day +21 post-HCT among children age 10 years or younger was also associated with NRM (P = .003; Figure 2). Results for this group of children are concordant with previous findings in mostly adult cohorts in which ST2 or TNFR1 values at day +21 post-HCT (before clinical signs of aGVHD) were associated with NRM. The other 2 biomarkers did not show significant differences between high and low levels when landmarks were analyzed individually in both age groups (Figure 2).
Cumulative incidence curves of NRM at post-HCT landmarks +7, +14, and +21 days, by age group, and by ST2, TNFR1, REG3α, and IL-6 values. Curves comparing 4 groups: (Ai, Bi, Ci) high vs low ST2 (above or below the median of 26 ng/mL); (Aii, Bii, Cii) high vs low TNFR1 (above and below the median of 3441 pg/mL); (Aiii, Biii, Ciii) high vs low REG3α (above and below the median of 25 ng/mL); and (Aiv, Biv, Civ) high vs low IL-6 (above and below the median of 26.5 pg/mL) in patients age 10 years or younger, and in those older than age 10 years (see supplemental Table 9).
Cumulative incidence curves of NRM at post-HCT landmarks +7, +14, and +21 days, by age group, and by ST2, TNFR1, REG3α, and IL-6 values. Curves comparing 4 groups: (Ai, Bi, Ci) high vs low ST2 (above or below the median of 26 ng/mL); (Aii, Bii, Cii) high vs low TNFR1 (above and below the median of 3441 pg/mL); (Aiii, Biii, Ciii) high vs low REG3α (above and below the median of 25 ng/mL); and (Aiv, Biv, Civ) high vs low IL-6 (above and below the median of 26.5 pg/mL) in patients age 10 years or younger, and in those older than age 10 years (see supplemental Table 9).
A CIF analysis was also performed on those with aGVHD grades 2 to 4, gut GVHD, and cGVHD as secondary outcomes. There was an association with ST2 on day +21 post-HCT and the cumulative incidence of aGVHD in those older than age 10 years (P = .04) (supplemental Figure 3). The curves for all other post-HCT landmarks and other biomarkers trended toward the right without reaching significance because of the small number of events in each subgroup (supplemental Figure 3). There was no association with any of the biomarkers and the cumulative incidence of gut GVHD, likely because of the even smaller numbers of gut GVHD compared with aGVHD grades 2 to 4 (n = 88 vs 157) (supplemental Figure 4). There was an association with the cumulative incidence of cGVHD in the age 10 years or younger group for high ST2 on day +14 (P = .01) and day +21 (P = .007) and IL-6 on day +21 (P = .03) (supplemental Figure 5).
Landmark analysis with post-HCT biomarkers for outcome prediction
The nonparametric analysis presented above was underpowered for 4 subgroups. We therefore evaluated whether biomarkers were associated with 1-year NRM using semiparametric proportional hazards models and including all the post-HCT landmarks (days +7, +14, and +21) (supplemental Table 3). The flowchart of this analysis is explained in the “Patients and methods” section and summarized in supplemental Figure 6. Among patients age 10 years or younger, the hazard of NRM was higher by 9.13-fold for those with ST2 values above the median compared with with those who had lower ST2 values (P = .0003). Among patients older than age 10 years, those with an ST2 value above the median had a 2.60-fold higher hazard of NRM (P = .021). These results indicate that ST2 is prognostic for NRM in both cohorts, but there was no significant association between ST2, age category, and NRM. There was also no significant association between ST2, landmark, and NRM. This means that there was no evidence that the association between the biomarker and the outcome changed over time starting from day +7 in both age populations. The hazard of NRM for the age 10 years or younger group was also 7.28-fold higher for patients with REG3α >25 ng/mL (P = .0022). Among patients older than age 10 years, those with high REG3α had a 2.57-fold higher hazard of NRM (P = .016). As with ST2, these associations started from day +7 and did not change over time in either population. High TNFR1 was also associated with a higher NRM HR for patients age 10 years or younger (P = .0007) but only trending in patients older than age 10 years (P = .064). IL-6 was not associated with NRM. Together, these data suggest that high ST2, REG3α, and TNFR1 are more prognostic for NRM in both age cohorts starting as early as day +7.
Although we focused on NRM, we also conducted the same landmark analysis on post-HCT time points for the secondary outcomes (aGVHD grades 2 to 4, gut GVHD, and cGVHD (supplemental Tables 4, 5, and 6, respectively). In the landmark analysis for patients with grades 2 to 4 aGVHD post-HCT, there was a trend for higher HR for ST2 in both age groups that did not reach significance, although high ST2 at day +21 was correlated with the cumulative incidence. Conversely, TNFR1 and IL-6, which did not correlate with cumulative incidence, do reach significance in the landmark analysis in both age groups (supplemental Table 4). In the gut GVHD post-HCT landmark analysis, there was no association with any of the biomarkers with the exception of REG3α. Indeed, although REG3α cumulative incidence functions were not significant, REG3α had a significant decreased HR on landmark analysis, likely driven by the day +7 post-HCT values (supplemental Table 5). In the cGVHD post-HCT landmark analysis, there was no association with any of the 4 biomarkers despite some association in the cumulative incidence (supplemental Table 6).
Biomarkers in pre-HCT samples and outcome by age group
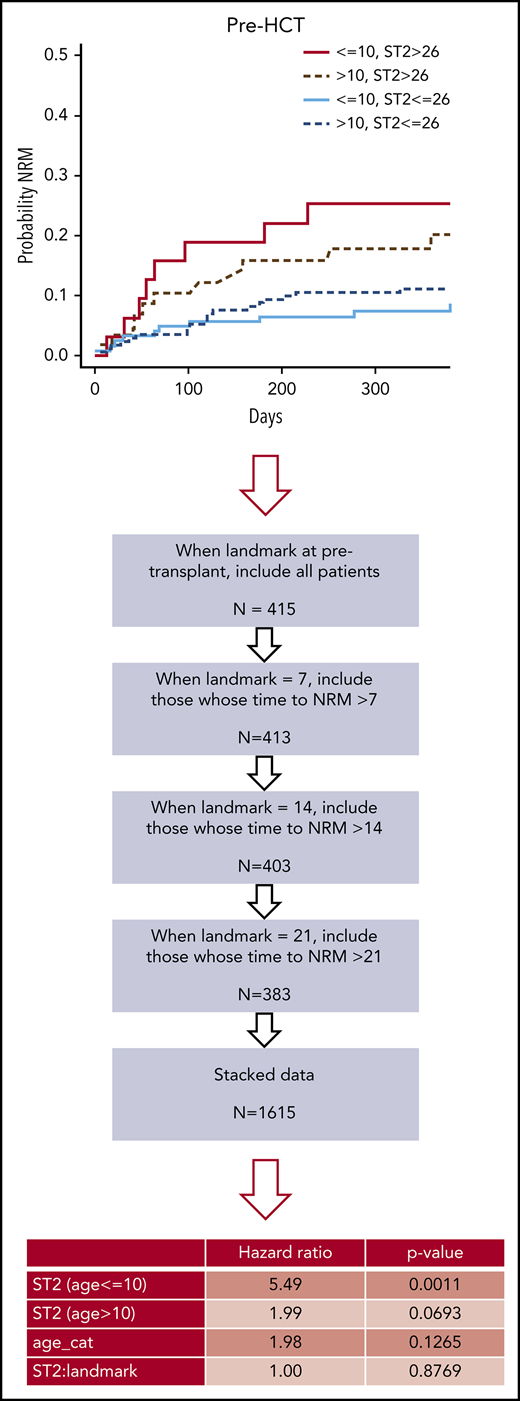
Early posttransplantation increases of ST2, REG3α, and TNFR1 have been associated with NRM in large cohorts5-8 ; however, there are no published reports of any association of pre-HCT levels with outcomes. Therefore, we sought to evaluate whether pre-HCT biomarkers can predict subsequent NRM and aGVHD. Interestingly, pre-HCT biomarker values revealed a significant difference in the probability of NRM between low and high ST2 groups but only in children age 10 years or younger (P = .014; Figure 3). High TNFR1 pre-HCT was significant in both age 10 years or younger (P = .014) and older than age 10 years (P = .019) groups (Figure 3). The two other biomarkers were not different in pre-HCT samples in either age group (Figure 3). There was no association with any of the 4 pre-HCT biomarkers and the cumulative incidence of aGVHD (supplemental Figure 7), gut GVHD (supplemental Figure 8) or cGVHD (supplemental Figure 9).
Cumulative incidence curves of NRM at the pre-HCT landmark of day –7, by age group, and by ST2, TNFR1, REG3α, and IL-6 values. Curves comparing 4 groups: (A) high vs low ST2 (above and below the median of 26 ng/mL); (B) high vs low TNFR1 (above and below the median of 3441 pg/mL); (C) high vs low REG3α (above and below the median of 25 ng/mL); and (D) high vs low IL-6 (above and below the median of 26.5 pg/mL) in patients age 10 years or younger and in those older than age 10 years (see supplemental Table 10).
Cumulative incidence curves of NRM at the pre-HCT landmark of day –7, by age group, and by ST2, TNFR1, REG3α, and IL-6 values. Curves comparing 4 groups: (A) high vs low ST2 (above and below the median of 26 ng/mL); (B) high vs low TNFR1 (above and below the median of 3441 pg/mL); (C) high vs low REG3α (above and below the median of 25 ng/mL); and (D) high vs low IL-6 (above and below the median of 26.5 pg/mL) in patients age 10 years or younger and in those older than age 10 years (see supplemental Table 10).
Landmark analysis with pre- and post-HCT biomarkers for NRM prediction
We next analyzed the 1615 values per biomarker from stacked landmarks −7, +7, +14, and +21 days. The flowchart and the results of this analysis are presented in supplemental Figure 10 and Table 2. The hazard of NRM is higher by 5.49-fold for those with an ST2 value above the median in the children age 10 years or younger (P = .001). Among patients older than age 10 years, those with an ST2 value above the median have a 1.99-fold higher hazard of NRM (P = .069). Again, there was no significant association between ST2, landmark, age, and NRM. REG3α, TNFR1, and IL-6 were not significantly associated with NRM when the analysis included pre- and post-HCT samples. These 4 biomarkers were also not correlated with each other because every correlation coefficient was ≤0.2 (supplemental Figure 11). Furthermore, the combination of markers did not improve the predictive accuracy for NRM. By using time-dependent prediction error estimates, we found that combining the 4 biomarkers did not improve prediction error further than ST2 alone (supplemental Table 7). Together, these data suggest that even high plasma levels of pre-HCT ST2 are prognostic of NRM particularly among children age 10 years or younger.
Cause-specific landmark analysis in the subgroup of patients with malignant diseases
We next evaluated the cause-specific covariates that are possibly responsible for the differences between high and low pre-HCT ST2 in the subgroup of patients with malignant diseases. We included the type of malignant disease, low or intermediate vs high disease risk index, and disease status at transplantation (first complete response [CR1] vs less than a CR1), relapsed, primary induction failure). Supplemental Table 8 shows the cause-specific model of NRM in which malignant disease type, disease risk index, and disease status at transplant (CR1 vs CR>1) were considered as potential covariates in this malignant group. HRs for high ST2 adjusted for these 3 covariates (malignant lymphoblastic diseases, high disease risk index, more than CR1) were all showing >20-fold increased hazard of NRM in children age 10 years or younger, although these HRs need to be cautiously considered because of the large standard error. Indeed, in this subset of patients with malignancies, there was a significant association between ST2, age category, and NRM. These results suggest that ST2 is more prognostic for NRM in patients age 10 years or younger in the subset of patients with malignancies after adjustment for malignant disease type, disease risk index, and disease status at transplant.
Multivariable analysis of biomarkers for NRM
For the landmark multivariable analysis, white and non-Hispanic vs not, malignant vs nonmalignant, graft source (marrow vs peripheral blood stem cells), and aGVHD prophylaxis (calcineurin inhibitors and methotrexate were the reference) were considered as potential covariates (Table 3). After all adjustments, the model revealed that high ST2 levels alone in children age 10 years or younger were significantly associated with NRM (P = .0056).
Discussion
In this study, we explored whether putative proteomic biomarkers previously assessed using HCT cohorts largely consisting of adults were applicable to children (defined as age 18 years or younger by the National Institutes of Health), with a particular focus on children younger than age 10 years. On the basis of the previous CIBMTR study showing that age older than 10 years was associated with worse survival,10 as well as the fact that the recovery of the immune system after HCT in children seems to be different from that of adults,21,22 we used age 10 years as a cutoff in our study. Indeed, in adults, the grafted T-cell pool is vulnerable to repertoire skewing toward reactions against alloantigens, a factor that may exacerbate GVHD. By contrast, in children, the antigen-driven, peripheral expansion phase of T-cell reconstitution is short-lived. An early dominance of the thymus-dependent phase may prevent expansion of alloreactive T-cell clones and may play a role in the reduced incidence of cGVHD in the pediatric population.10
Among the novel features in our study is the reliance on a prospective contemporary (2013 to 2018) and large multicenter clinical-biologic cohort of more than 400 HCT recipients, 70% of whom were children age 18 years or younger. This allowed us to assess the current trends in HCT across 6 major US pediatric centers. The first demographic finding was that the number of non-white and non-Hispanic recipients was significantly higher in the age 10 years or younger group and across the age groups compared with historical cohorts.23,24 This increase in mixed race seen in the younger group follows the trend for the general US population.25 Not surprisingly, the number of patients with nonmalignant indications for HCT is much higher (close to 50%) in the age 10 years or younger group. For malignant indications, lymphoblastic leukemia was overrepresented in the age 10 years or younger group. Particularly striking is the trend toward a higher percentage of high disease risk index at time of transplantation in the age 10 years or younger group. The graft source was marrow in >80% for the age 10 years or younger group and >50% in the older age group, suggesting adherence to the recommendations resulting from the phase 3 multicenter randomized trial conducted by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN #0201) that compared HCT with peripheral-blood stem cells vs bone marrow from unrelated donors. The study revealed a similar incidence of aGVHD but a significantly reduced incidence of cGVHD in the marrow group. Of interest, 1 center used cyclophosphamide post-HCT in conjunction with marrow graft. This practice may have offset the expected higher risk of aGVHD in a population with mixed race and high disease risk index. aGVHD prophylaxis differed by center. For example, post-HCT cyclophosphamide was mostly used at the Johns Hopkins University School of Medicine), which was a participating center that had only pediatric patients. Antithymocyte globulin and alemtuzumab were used mostly in the younger age group, likely for nonmalignant HCT indications. Sirolimus was used only in the older than age 10 years group.
With the exception of a single-center study,26 our multicenter cohort study is the first to examine the relevance of promising biomarkers in pre-HCT samples. All samples were uniformly tested using the same laboratory protocols that have been established by our laboratory and currently in use in BMT CTN trials.2-5,11-14 We have relied on novel statistical methods to address our scientific aims. These include the cause-specific landmark approach corresponding to a multistate competing risks model. We chose some opportune early landmarks (pre-HCT, and days +7, +14, and +21 post-HCT) and performed a sequence of regression analyses at these time points. We assumed separate semiparametric Cox regression models for the cause-specific hazards. The advantage of this approach is to provide predictions of how a patient’s prognosis may change over time without imposing strong parametric assumptions about the shape of the cause-specific hazards. It can be used to estimate and model clinically relevant quantities, as well as to provide information on how patients’ current state affects their subsequent prognosis. This approach is well suited for prognostic biomarkers. The limitation is that it does require substantial detail in data collection and a large sample size because of the number of transitions between states that need to be captured.27,28 We found that, despite low rates of NRM at 1 year in this mostly pediatric cohort, increased levels of plasma proteins remained prognostic for adverse outcomes. Consistently in both age groups, increases in ST2, TNFR1, and REG3α at +7, +14, and +21 days posttransplantation were significantly associated with greater cumulative incidence of NRM. Previous studies of plasma protein biomarkers in large adult cohorts at predetermined time points during the first month after transplantation have yielded associations between levels of ST2, TNFR1, and REG3α, and subsequent occurrence of NRM and/or aGVHD. A recent large multicenter study of 1287 adult patients revealed that increased day +7 ST2 and REG3α levels were associated with greater risk for 6-month NRM, GVHD-related mortality, and gastrointestinal GVHD.5,7,8
It is possible that GVHD and many cases of NRM are direct corollaries of a dysregulated immune system, which is what many of these biomarker proteins are measuring. However, substantial preclinical studies have been performed to explore the role of ST2 and REG3α in aGVHD. Soluble ST2 (sST2) (the form measured by ELISA) has been shown to be secreted by intestinal stromal cells and CD4 and CD8 T cells producing interferon-γ and IL-17.29 sST2 acts as the decoy receptor for IL-33, limiting its availability to cytoprotective regulatory T cells expressing the transmembrane molecule form of ST2.29,30 Furthermore, blockade of sST2 in the peritransplantation period with a neutralizing monoclonal antibody reduced GVHD severity and mortality by increasing the availability of IL-33 to cytoprotective T cells.29 REG3α functions as an antimicrobial protein that protects the gastrointestinal epithelium during inflammation, including in GVHD.31 Recently, single nucleotide polymorphisms in the IL1RL1 region have been associated with higher sST2 levels in the donor and an increase in the cumulative incidence of both aGVHD and infection-related deaths.32
We also show that ST2 proteomic levels in recipients pre-HCT are associated with NRM and may reflect predisposition for inflammatory reactions in them. One possible explanation is that there was an association with malignant disease type (lymphoblastic leukemia) and disease burden, as shown in the cause-specific landmark analysis of patients with malignant diseases. However, it is challenging to confirm this inference because of the lack of data in our de-identified database.
A remarkable finding in our study was that ST2 measured before HCT was prognostic for 1-year NRM and severe aGVHD in the younger age group, independent of all other covariates. The ability to identify patients at high risk for NRM and GVHD before their transplant and intense treatment course has potential important therapeutic implications, including more stringent monitoring and preventive care. Indeed, given the pre-HCT prognosis for longer-term risk, an important issue is whether this risk is modifiable with more intensive monitoring, individualized or less aggressive conditioning, choice of donor and graft source, prophylactic immunosuppressive treatment, or other novel interventions (ie, risk-adapted therapy). On the basis of the cause-specific analysis in the malignant group (supplemental Table 8), another consideration could be to bring children younger than age 10 years who are to receive a transplantation for malignant conditions to HCT earlier so they do not develop a higher inflammatory state before transplantation. The patients who received a transplantation for nonmalignant conditions who had high ST2 before HCT were also at higher risk of NRM. They might also benefit from an early transplantation before too many complications (particularly infections) arise because of their disease. Furthermore, low levels of ST2 could identify patients that can safely tolerate a myeloablative regimen. Another strength of our study is that we defined a cutoff of high vs low ST2 based on day +21 levels that consistently identified a group of patients at high risk for NRM. Future studies should prospectively and serially evaluate these biomarkers beginning before HCT to help facilitate personalized, risk-adapted HCT strategies for children.
We conclude that a noninvasive ST2 assay performed as early as 7 days before HCT is a promising approach for identifying children age 10 years or younger who are at high risk for NRM. Our results suggest that pre-HCT interventions and improved monitoring strategies might decrease the risk of death in this young population.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the clinicians at all of the institutions that helped accrue samples, and all of the data managers for excellent management of the database and biobank (Divyesh Kukadiya, Johnetta Nakibu, JoAnn Lorenzo, Anise Marshall, Marlen Dinu, Lauren Leonard, Andrea Demarsh, Sophie Silverstein, Magda Polanczyk, Megan Petrycki, Kara Jodry, Rose Case, and Lindsey Elmore), the Indiana University Children’s Clinical Research Center laboratory managers (Jenny Then, Brian Ashmore, and Khadijeh Bijangi-Vishehsaraei), and the S.P. laboratory for help with generating all the biomarkers values on thousands of samples.
This work was supported by grants from the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD074587) and the National Cancer Institute (R01CA168814), the Leukemia and Lymphoma Society (1293-15), and the Lilly Physician Scientist Initiative Award.
Authorship
Contribution: C.M.R. performed proteomics analysis, participated in research discussions, and wrote the paper; F.P., H.L., and G.B. served as study statisticians, conceived the statistical plan, and wrote the manuscript; K.R.C., R.K., P.A.C., C.D., D.A.J., C.M.B., C.R.Y.C., S.S.F., and J.R. contributed to patient accrual, clinical data collection, quality assurance, research discussion, and writing the manuscript; A.M. built and maintained the biorepository, participated in research discussions, and wrote the paper; and S.H. and S.P. conceived and planned the study design, built and maintained the biorepository and biobank, supervised proteomics experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: K.R.C. is on the advisory board of Jazz Pharmaceuticals. C.R.Y.C. is co-founder of Mana Therapeutics. S.P. is an inventor on patent US-13/573 766 (Methods of detection of graft-versus-host disease). The remaining authors declare no competing financial interests.
Correspondence: Sophie Paczesny, Indiana University School of Medicine, 1044 W Walnut St, Room 425, Indianapolis, IN 46202; e-mail: sophpacz@iu.edu.