Introduction
FLT3 receptor gene has been reported to be mutated in about 30% of AML, with two different kinds of mutations identified: in-frame duplications within the juxtamembrane region (FLT3-ITD) and point mutations in the tyrosine kinase domain (FLT3-TKD). In term of prognosis, the proper role of these mutations is still debated. Moreover, FLT3 mutations are often subjected to clonal evolution, thus how to properly monitor FLT3 mutated clones, evaluate minimal residual disease, manage FLT3 inhibitors (FLT3i) are only some of the open issues.
FLAM is an observational study involving FLT3 positive AMLs to gain clinical and molecular data useful to ameliorate real-life physicians' management of this disease. Here we report the results of a preliminary analysis of the retrospective phase of the study.
Methods
The retrospective phase of FLAM multi-center observational study enrolled each AML patient treated in 33 participating Italian centers detected to carry a FLT3 mutation since 2012. Clinical and molecular data were collected in accordance with GCP and Helsinki declaration in electronic case report forms.
Results
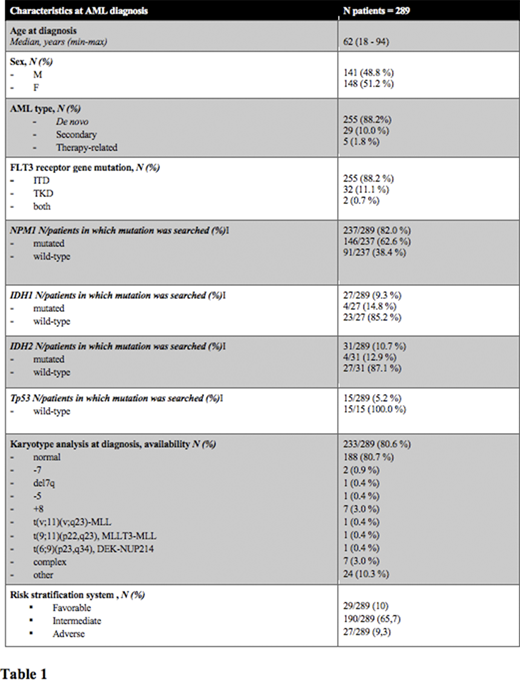
At data cut-off , 1st of July 2020, 289 patients with FLT3 mutation at diagnosis were enrolled in the retrospective phase of FLAM study, being evaluable in this analysis, with a median age at diagnosis of 62 years (min-max: 18-94) and a M:F ratio of 141/148 (Patients' characteristics are summarized in table 1). 29 out 289 (10 %) patients had a low risk AML, 190/289 (65,7 %) had an intermediate risk AML and 27/289 (9,3 %) patients had a high risk AML, according to ELN 2017 risk stratification or ELN 2010 in case of allelic ratio unavailability (43 patients had no available ELN risk at baseline). A more frequent association between FLT3-ITD and normal karyotype and between FLT3-TKD mutation and other cytogenetic alterations not conferring a favorable/adverse risk has been observed (p = 0.045).
Among the study population, 255/289 (88 %) patients carried a FLT3-ITD, 32/289 (11 %) a FLT3-TKD point mutation and 2/289 (1 %) patients both mutations. Capillary electrophoresis has been the technical method used to investigate FLT3-ITD in 163 of 226 (72 %) patients with information on the method used, while Sanger Sequencing in 47 out of 226 (21 %) patients and Next generation sequencing (NGS) in 16 out of 226 (7 %) patients. Overall, NGS has been adopted to investigate FLT3 gene status in 18 out of 259 patients (7 %). FLT3-ITD allelic ratio was available in 62 of 257 (24 %) ITD patients and was greater than or equal to 0.5 in 35/62 (56 %) patients.
During patients' follow-up, 19/289 (7 %) patients affected by a FLT3 positive AML at diagnosis underwent a disease clonal evolution with a FLT3 negative AML progression or relapse.
Regarding treatment options in FLT3-AML, in our cohort FLT3i were administered as first-line of therapy in only 36/289 (13 %) patients, always in a combination, of which in 26/36 (72.2 %) with intensive chemotherapy. As expected, intensive chemotherapy represented the induction regimen in the majority of the patients (211/289, 73 %). On the other hand, FLT3i were administered as rescue therapy in 62/171 (36 %) cases (47/62 single-agent and in 15/62 in combination) and as re-induction therapy in 22/80 (28 %) cases (10/22 single-agent and 12/22 in combination). Overall, a FLT3i has been administered as single-agent 81 times, of which Gilteritinib was the most frequently used (56/81, 69,1 %), followed by quizartinib (18/81, 22,2 %). Among the 52 documented combinations of a FLT3i with other drugs, particularly noteworthy is the administration of Sorafenib, in 20/52 (39 %) cases in this real-life study. Lastly, nine out of 289 (3 %) patients received a FLT3i as maintenance therapy after HSCT.
Data regarding the correlation among the different regimens, with a special focus on FLT3i, other molecular features and response/survival are currently under analysis.
Conclusions
These data coming from a preliminary analysis portray the state of a large multi-center retrospective cohort of FLT3-positive AML patients treated in Italy between 2012 and 2020, including interesting insights regarding technical methods used to characterize the disease and the therapeutic scenario in which FLT3 inhibitors have been developed. Further safety and effectiveness data may reveal beneficial to ameliorate physicians' real-life clinical practice.
Acknowledgements: work supported by Daiichi-Sankyo.
Martelli:Amgen: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Rigolin:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Fracchiolla:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Speakers Bureau; ABBVIE: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses, Speakers Bureau. Bocchia:Incyte: Honoraria; CELGENE: Honoraria. Todisco:Jannsen, Abbvie, Jazz: Membership on an entity's Board of Directors or advisory committees. Papayannidis:Abbvie, Janssen, Novartis, Amgen, Pfizer: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.
Sorafenib is a registered drug for the treatment of hepatocellular carcinoma. Due to its multi-targeted tirosine kinase inhibitor effect it demonstrated efficacy in FLT3-AML when administered as an off-label prescription.
Author notes
Asterisk with author names denotes non-ASH members.