Key Points
De novo immune response to hepatitis B vaccine was nearly absent in CLL patients on BTKi and impaired in treatment-naïve patients.
Recall immune response to zoster vaccine was not significantly different between CLL patients on BTKi and treatment-naïve patients.
Abstract
Vaccinations are effective in preventing infections; however, it is unknown if patients with chronic lymphocytic leukemia (CLL) who are treatment naïve (TN) or receiving Bruton tyrosine kinase inhibitors (BTKi's) respond to novel adjuvanted vaccines. Understanding the effect of BTKi's on humoral immunity is timely because BTKi's are widely used and vaccination against coronavirus disease 2019 is urgently needed. In 2 open-label, single-arm clinical trials, we measured the effect of BTKi's on de novo immune response against recombinant hepatitis B vaccine (HepB-CpG) and recall response against recombinant zoster vaccine (RZV) in CLL patients who were TN or on BTKi. The primary end point was serologic response to HepB-CpG (anti-hepatitis B surface antibodies ≥10 mIU/mL) and RZV (≥fourfold increase in anti-glycoprotein E). The response rate to HepB-CpG was lower in patients on BTKi (3.8%; 95% confidence interval [CI], 0.7-18.9) than patients who were TN (28.1%; 95% CI, 15.6-45.4; P = .017). In contrast, the response rate to RZV did not differ significantly between the BTKi (41.5%; 95% CI, 27.8-56.6) and TN cohorts (59.1%; 95% CI, 38.7-76.7; P = .2). BTKi's were associated with a decreased de novo immune response following HepB-CpG, whereas recall immune response following RZV was not significantly affected by BTKi therapy. These trials were registered at www.clinicaltrials.gov as #NCT03685708 (Hep-CpG) and #NCT03702231 (RZV).
Introduction
Infections are a major cause of morbidity for patients with chronic lymphocytic leukemia (CLL).1 Vaccines are effective in preventing infections; unfortunately, vaccine responses in patients with CLL have been suboptimal.2 It is unknown whether novel adjuvanted vaccines can induce superior immune responses in patients with CLL who are treatment naïve (TN) or receiving Bruton tyrosine kinase inhibitors (BTKi's).
BTK is critical for B-cell receptor signaling and humoral immunity. BTKi's are widely used to treat CLL.3,4 Among patients with CLL on BTKi therapy, antibody-mediated responses to influenza vaccination are detected in 7% to 26%.5,6 Humoral immunity against influenza is, at least in part, dependent upon recognition of recall antigens from prior infection or vaccination.7 In contrast, protection against other viruses, such as severe acute respiratory syndrome coronavirus 2, requires the induction of a de novo immune response.8
The impact of BTKi on vaccine responses has potential implications for vaccination strategies for an increasing number of patients with CLL receiving this therapy. To test the antibody-mediated response to novel antigens, we conducted a phase 2 clinical trial of recombinant adjuvanted hepatitis B vaccine (HepB-CpG) in patients with TN CLL or those on BTKi therapy. A concurrent clinical trial of recombinant adjuvanted shingles vaccine (RZV) evaluated the recall response to varicella-zoster virus (VZV).
Methods
These 2 prospective, open-label, phase 2 studies investigated the efficacy of HepB-CpG (HEPLISAV-B, Dynavax Technologies Corp) and RZV (SHINGRIX, GlaxoSmithKline Biologicals) in patients with TN CLL, or patients receiving a BTKi (ibrutinib or acalabrutinib) for ≥6 months as frontline treatment or for relapsed disease. Patients received HepB-CpG or RZV individually, in combination, or sequentially. Key exclusion criteria were concomitant immunoglobulin replacement or immunosuppressive therapy (eg, corticosteroids). Patients receiving RZV must not have had symptomatic VZV infection, exposure to live shingles vaccine within the past 1 year, or prior RZV vaccinations. Patients receiving HepB-CpG must not have had previous hepatitis B infection or immunization confirmed by negative hepatitis B surface antibodies (anti-HBs) and hepatitis B core antibodies. Both studies were approved by the National Institutes of Health Intramural Institutional Review Board. All patients provided informed consent.
HepB-CpG and/or RZV were given at 0 and 3 months via intramuscular injection. Serologic titers were measured at baseline and 6 months from the first vaccine administration. The primary end point for HepB-CpG was the rate of hepatitis B seroprotective titer achievement (anti-HBs blood titer ≥10 mIU/mL). The primary end point for RZV was the rate of seroconversion (≥fourfold rise in VZV anti-glycoprotein E [anti-gE] blood immunoglobulin G titer) measured via luciferase immunoprecipitation assay.9 The serologic response end points for HepB-CpG and RZV are consistent with vaccine trials conducted in the general population.10,11 All subjects completed an adverse event (AE) diary documenting local or systemic AEs that started within 7 days after each vaccine dose. If subjects received 2 vaccines concomitantly, systemic AEs were attributed to both vaccines and local AEs were attributed to the vaccine that was given at the corresponding site. We used descriptive statistics to report the rate of response to HepB-CpG and RZV. Mann-Whitney test and Fisher’s exact test were used to compare titers and response rates between different groups, respectively.
Because of the low response rate to HepB-CpG among patients on BTKi, we calculated that the conditional power of rejecting the null hypothesis (response rate ≤10%) was <1% if the study were to continue to the planned sample size of 54 patients. Therefore, the BTKi cohort of the HepB-CpG trial was stopped early for futility; final results are presented. Preliminary analyses of the HepB-CpG TN cohort and the RZV trial were performed to compare differences in de novo and recall humoral immune responses between BTKi-treated and patients who wereTN.
Results and discussion
Vaccine response was evaluable in 58 patients receiving HepB-CpG and 63 patients receiving RZV. Baseline characteristics and demographic information are summarized in Table 1. One patient withdrew consent after receiving the first dose of both vaccines. One patient interrupted treatment with ibrutinib for >4 weeks and was excluded from the efficacy analysis.
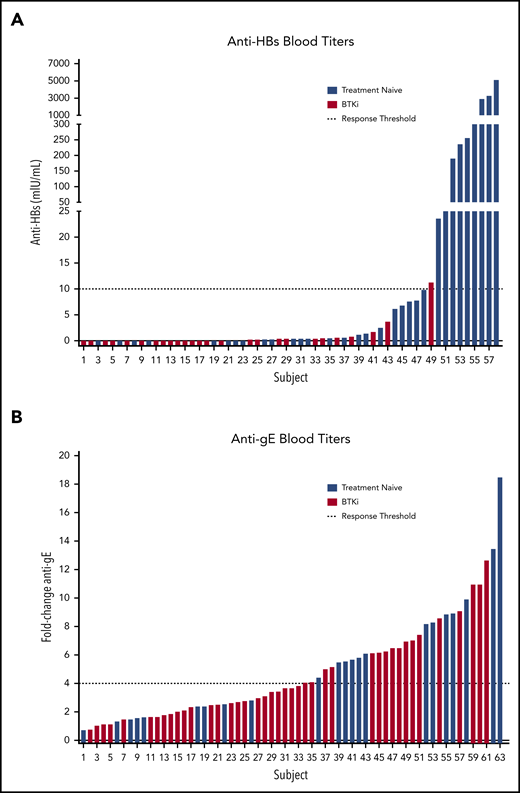
Only 1 (3.8%; 95% confidence interval [CI], 0.7-18.9) patient on BTKi responded, whereas 9 (28.1%; 95% CI, 15.6-45.4) patients who were TN responded to HepB-CpG (P = .017) (Figure 1). At 6 months, the median anti-HBs titer was 0.05 mIU/mL (interquartile range [IQR], 0.00-0.43 mIU/mL) in the BTKi group and 1.30 mIU/mL (IQR, 0.15-43.78 mIU/mL) in patients who were TN.
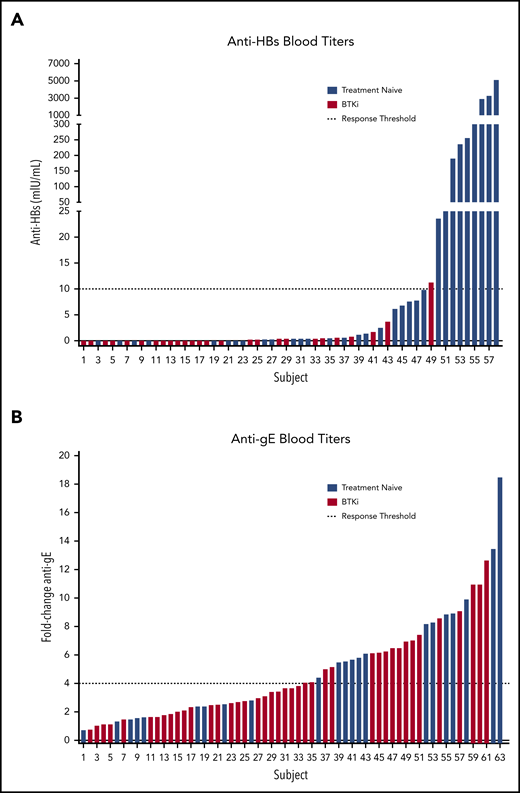
Evaluation of immunogenicity. (A) HepB-CpG and (B) RZV. Dashed lines indicate response threshold (HepB-CpG: anti-HBs ≥10 mIU/mL; RZV: ≥fourfold increase in anti-gE titer).
Evaluation of immunogenicity. (A) HepB-CpG and (B) RZV. Dashed lines indicate response threshold (HepB-CpG: anti-HBs ≥10 mIU/mL; RZV: ≥fourfold increase in anti-gE titer).
BTKi therapy did not significantly affect the rate of response to RZV. Seroconversion was detected in 17 (41.5%; 95% CI, 27.8-56.6) patients on BTKi and 13 (59.1%; 95% CI, 38.7-76.7) patients who were TN (P = .2) (Figure 1). Similarly, there was no difference between serum anti-gE antibody titer at baseline or at 6 months between patients who were BTKi-treated and TN (P = .5 at baseline; P = .7 at 6 months). Response to RZV was not significantly different between patients receiving BTKi as frontline treatment and for relapsed disease (P = .8) or between patients receiving ibrutinib (n = 9) and acalabrutinib (n = 8, P = .8). The rate of response to HepB-CpG or RZV was comparable in patients who received either vaccine alone or both vaccines concomitantly (P = .4 for HepB-CpG; P = .5 for RZV).
Most AEs were grade 1-2 in severity (supplemental Table 1 on the Blood Web site). The most common local AE was injection site pain (85.1% HepB-CpG; 97.3% RZV) and the most common systemic AE was myalgia (41.9% HepB-CpG; 51.3% RZV). No treatment-related serious AEs occurred.
These studies confirm that HepB-CpG and RZV administration is safe for patients with CLL, with similar rates of AEs compared with healthy individuals.10,12 However, response rates to HepB-CpG and RZV exceed 95% in the general population.10,12 The HepB-CpG response rate of 28.1% in TN CLL patients in this study is consistent with prior investigations of the pneumococcal conjugated vaccine (PCV13) and pneumococcal polysaccharide vaccine (PPSV23) that reported response rates between 20% and 40%.13-15 These results highlight that vaccine responses are impaired in TN CLL patients, even with novel adjuvanted formulations. BTKi's, a standard treatment in B-cell malignancies, were associated with a near absence of de novo humoral immune response to HepB-CpG, whereas recall response to RZV was largely preserved.
BTKi's are currently being investigated to treat coronavirus disease 2019-related respiratory failure and associated complications (NCT04375397, NCT04346199). Although the immunomodulatory effects of BTKi's may prove beneficial in reducing cytokine-mediated organ damage in severely ill patients, our study suggests that BTKi's may decrease antibody formation in response to novel antigens.
This study has several limitations. First, the number of participants is small in the context of vaccine studies. Second, although the virtual absence of a de novo humoral immune response to HepB-CpG on BTKi therapy is striking, patients with TN CLL could be more immunocompetent because of less advanced disease, thereby permitting more effective immune responses. Third, the generalizability of our findings, particularly for patients without immunocompromising conditions, requires confirmation.
In summary, CLL patients have impaired vaccine responses compared with the general population. The humoral immune response to novel antigens is abrogated by BTKi's, whereas response to recall antigens appears preserved. Vaccination against novel antigens would ideally be scheduled early in the disease course and before starting a BTKi. Both patients who were TN CLL and patients receiving a BTKi can derive benefit from RZV vaccination. Ongoing efforts are directed at improving vaccination strategies for patients with CLL.
For original data, please contact wiestnea@nhlbi.nih.gov.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in this trial and their families; Lola Ulomi for data management; Adriana Byrnes, Devika Abraham. and Ovsanna Melikyan for protocol support; and the National Institutes of Health, Office of Clinical Director for administrative support.
Funding for this work was provided by National Institutes of Health National Heart, Lung, and Blood Institute (HL006070-10) and an Intramural Program of the National Heart, Lung and Blood Institute and the National Institute of Allergy and Infectious Diseases. Additional funding came from the American Society of Hematology Scholar Award (I.E.A.). Pharmacyclics LLC, an Abbvie company, and Acerta Pharma, a member of the Astra-Zeneca group, provided BTKi's and research support. All data were collected, stored, and analyzed by the investigators. Corresponding authors have full access to data and human samples collected from the study and have final responsibility for the content of the manuscript.
Authorship
Contribution: C.P. was principal investigator and prepared the manuscript; M.A.A. and J.I.C. analyzed laboratory samples; E.M.G. processed laboratory samples; X.T. performed statistical analysis; S.S. and P.N. provided research support and study coordination; I.E.A., G.E.M., C.H., J.L., J.S., C.P., A.W., and C.S. evaluated patients on the study; C.P., A.W., and C.S. conceptualized the studies and prepared the first draft of the manuscript; and all authors reviewed the final draft
Conflict-of-interest disclosure: A.W. received research support from Pharmacyclics LLC, an AbbVie company; Acerta Pharma, a member of the Astra-Zeneca group; Merck; Nurix; Verastem; and Genmab. The remaining authors declare no competing financial interests.
Correspondence: Adrian Wiestner, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, 10 Center Dr, CRC 3-5150, Bethesda, MD 20892;e-mail: wiestnera@mail.nih.gov; and Clare Sun, 10 Center Dr, CRC 3-5132, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD 20892; e-mail: clare.sun@nih.gov.