von Willebrand factor (VWF) deficiency has important hemostatic consequences but unraveling its genetic determinants has been a significant challenge. In this issue of Blood, Sadler and colleagues report that rare nonsynonymous (protein-coding) variants in VWF show important and significant association with the severity of VWF deficiency.1 The authors evaluated this by sequencing the coding regions of the VWF gene for unrelated persons with low VWF and von Willebrand disease (VWD), and normal subjects, followed by testing whether the presence of rare protein-coding variants is predictive of lower VWF antigen levels.
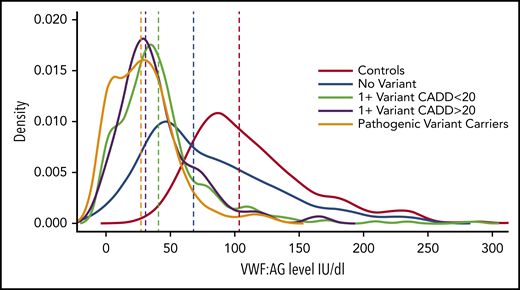
VWF factor levels among groups with different types of rare nonsynonymous VWF variants. The distributions of VWF antigen levels (vertical lines show medians) in normal subjects are compared with those of groups of subjects with VWD or low VWF who carry pathogenic variants, predicted pathogenic variants (1 + CADD >20), variants with low pathogenicity (1 + CADD <20), or no variants. CADD, combined annotation-dependent depletion. See Figure 3 in the article by Sadler et al that begins on page 3277.
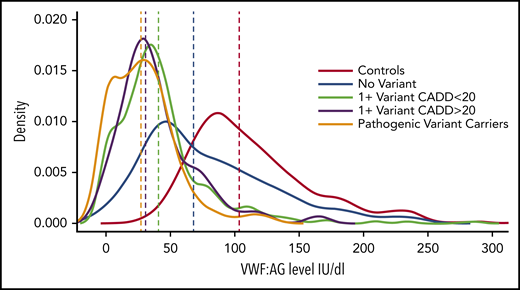
VWF factor levels among groups with different types of rare nonsynonymous VWF variants. The distributions of VWF antigen levels (vertical lines show medians) in normal subjects are compared with those of groups of subjects with VWD or low VWF who carry pathogenic variants, predicted pathogenic variants (1 + CADD >20), variants with low pathogenicity (1 + CADD <20), or no variants. CADD, combined annotation-dependent depletion. See Figure 3 in the article by Sadler et al that begins on page 3277.
Sadler et al provide important new knowledge of the determinants of VWF antigen levels, which has emerged to be a complex trait, influenced by VWF and additional genes and acquired factors.2-4 There are several noteworthy findings. First, Sadler and colleagues identified a relationship between the burden (or number) of rare, nonsynonymous VWF variants and the severity of VWF deficiency that was significant across all types of VWD. They noted that patients with type 3 VWD (who have the most severe VWF deficiency) had the greatest burden of such variants, although all groups, including those with type 1 or 2 VWD and low VWF, had more rare nonsynonymous variants than normal subjects. It is interesting that in none of the cases did the patients have more than 2 pathogenic or probable pathogenic variants. In Figure 3 Sadler et al provide a helpful illustration of the distribution of VWF antigen levels among normal subjects vs groups with VWF deficiency that do or do not carry rare, nonsynonymous VWF variants. It illustrates that the lowest VWF levels are in carriers of rare variants with known or predicted pathogenicity (based on high pathogenicity scores), and the less severe effects of carrying rare variants with low pathogenicity scores (see figure).
Many rare protein-coding sequence variants are of recent origin,5 and this may explain the considerable heterogeneity in rare VWF sequence variants that Sadler and colleagues found in their study. It is possible that additional determinants of VWF levels will be identified by looking beyond VWF for associations between VWF levels and rare protein-coding variants. Indeed, exploring for such relationships by a genome-wide approach has yielded important information for other human traits and diseases in 2 noteworthy studies.6,7 Given the findings of Sadler et al and the considerable evidence that other genes influence VWF levels,2,8 it would be particularly interesting to use whole-exome or -genome sequencing to investigate whether rare protein-coding variants (or other mutations) in those other genes would help predict the severity of VWF deficiency among persons with VWD or low VWF.
There are some intriguing questions about causation vs association that future studies could address, given the interesting findings of Sadler et al. For example, family studies of VWD and low VWF, that include index cases with multiple, rare, protein-coding VWF variants, would help determine whether the pathogenic or probable pathogenic variants are commonly coinherited with rare nonpathogenic variants that could be markers of a “disease allele.” This determination may explain why rare protein-coding variants with low pathogenicity scores show significant association with low VWF levels, whereas variants with known or predicted pathogenicity are associated with even lower VWF levels. In addition, family studies would help address whether VWF levels are significantly lower when both copies of VWF contain protein-coding variants with known or probable pathogenicity. The copy numbers of some other VWF variants have already been established as influencing VWF levels.4 It would also be interesting to directly test several of the rare nonsynonymous VWF variants (with high vs low pathogenicity scores) that persons with VWD and low VWF carry, to determine their effects on VWF biosynthesis and secretion, as there is evidence that the more common VWF deficiencies are caused by reduced VWF synthesis and/or constitutive secretion.3
Do the new findings have implications for how physicians diagnose or treat VWD and low VWF? Laboratories that perform VWF sequencing for diagnostic purposes should incorporate the findings of Sadler et al when reporting on the presence of rare nonsynonymous variants. New guidance on VWD diagnosis was recently published that considered current evidence, real and potential benefits and harms, patient values and preferences, and practical issues that have impacts on diagnosis, treatment, and access to care.9 The added value of testing persons with bleeding and confirmed VWF deficiency for rare nonsynonymous variants for purposes other than research awaits further exploration. It is noteworthy that Sadler and colleagues estimated that rare nonsynonymous variants in the VWF gene independently predict ∼16% of VWF antigen levels, which is an important but lesser effect than ABO blood group.2 Other inherited traits, age, and comorbidities also influence VWF levels in both health and disease.2,10
Conflict-of-interest disclosure: The author declares no competing financial interests.