Chronic venous disease (CVD), which includes chronic venous insufficiency (CVI), a common chronic condition with one of its mechanisms involving calf muscle pump dysfunction, is a potential, but poorly appreciated, risk factor for venous thromboembolism (VTE). In this issue of Blood, Houghton et al identified patients in Olmsted County, Minnesota without a history of VTE who had undergone evaluation of calf muscle function by plethysmography (see figure) over a 17-year period.1 Only those with plethysmographically determined normal venous outflow bilaterally (indicating no obstructive deep vein thrombosis [DVT]) were included. Patients with unilateral calf muscle pump dysfunction were more likely to develop DVT, and any calf muscle pump dysfunction was associated with higher mortality.
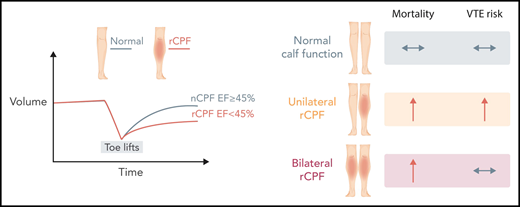
Air plethysmography findings in normal calf muscle pump function (nCPF) compared with reduced calf muscle pump function (rCPF) (left panel). Outcomes of mortality and VTE risk by calf pump function status (right panel). EF, ejection fraction. Professional illustration by Somersault18:24.
Air plethysmography findings in normal calf muscle pump function (nCPF) compared with reduced calf muscle pump function (rCPF) (left panel). Outcomes of mortality and VTE risk by calf pump function status (right panel). EF, ejection fraction. Professional illustration by Somersault18:24.
CVD (including CVI) is a multifaceted disease, with 3 basic pathophysiologic states existing in isolation or in any combination: valvular insufficiency, venous obstruction inside or external to the involved veins, and calf muscle pump dysfunction. The physiologic hallmark is venous hypertension that is manifested by diverse, but easily recognizable, clinical signs ranging in severity from small reticular veins, varicosities, and edema to hyperpigmentation, lipodermatosclerosis, and ulceration.2 CVI may be primary or secondary to events such as previous DVT. When caused by a previous DVT, CVI is called postthrombotic syndrome. CVD is ubiquitous in the aging population, with a prevalence of clinically significant disease as high as 64%.2,3
Among patients with CVI, the risk of developing venous thromboembolic events, including DVTs and pulmonary emboli (PEs), is incompletely understood. Multiple observational studies found that the diagnosis of CVI is associated with VTE; as an example, among 1272 medical outpatients, CVI was present in 70% of DVT cases compared with 41.4% of controls (odds ratio, 4.45, 95% confidence interval [95% CI], 3.10-6.38).4 Varicose veins, a clinical manifestation of CVD, are a well-established risk factor for VTE.5 What is less clearly understood is the precise role of the 3 pathophysiologies in contributing to VTE risk, because CVD is a heterogenous diagnosis. For example, primary valvular reflux has only recently been identified as a novel risk factor for VTE. In a nested case-control study, outpatients with DVT were 4.7 times more likely to have primary valvular reflux.6 Despite the established precedent for a role for venous hypertension, in general, as a risk factor for VTE, the role of calf muscle function, in particular, has not been studied.
Houghton et al found that >60% of patients (mean age, 64 years; 69% female) evaluated had reduced calf muscle pump function (rCPF), either unilaterally or bilaterally (see figure). After a mean follow-up of 11.7 years, patients with bilateral rCPF compared with bilateral normal calf muscle pump function (CPF) demonstrated an increased incidence of VTE (hazard ratio [HR], 2.0; 95% CI, 1.2-3.4), DVT only (HR, 2.2; 95% CI, 1.1-4.2), and proximal DVT, as well as higher mortality, but not PE alone. This relationship was more pronounced when individuals with a possible indication for antiplatelet agents were excluded. In a per-leg analysis, the cumulative incidence of ipsilateral DVT was higher in legs with rCPF (HR, 2.0; 95% CI, 1.20-3.30); after adjustment, the HR for ipsilateral DVT was 1.71 (95% CI, 1.03-2.84). After multivariable adjustments for age, sex, body mass index, and Charlson Comorbidity Index, bilateral rCPF was not an independent risk factor for VTE or DVT alone. However, when ipsilateral DVT was the outcome while examining each leg individually, rCPF remained an independent risk factor. Additionally, unilateral and bilateral rCPF compared with normal CPF were independent predictors of all-cause mortality.
This study provides us with 2 important incremental insights: one is related to the clinical care of patients, and the other is a better understanding of the mechanistic underpinnings of DVT physiology in relation to calf muscle pump function. Individual risk assessment is a well-established method to decrease VTE incidence among surgical and medical patients.7 Although >40 risk factors have been included in risk-assessment models, calf muscle pump dysfunction per se is not among them. Related factors, such as immobility or plaster casting with the ankle joint in equinus, are included, because blood stasis is a component of Virchow’s triad. The current study suggests that, in addition to the concept of simple immobility, we need to consider how efficiently the leg pumps and empties blood. Importantly, calf muscle pump function can be modified with appropriate exercise,8 raising the potential that this risk factor can be reduced. Second, the mechanisms controlling CVD and VTE risk are poorly understood. The current study isolates 1 of the 3 major pathophysiologies of CVD and establishes it as a risk factor. More concentrated efforts to study calf muscle pump function in relation to proximal venous hemodynamics may now allow us a more nuanced understanding of the tipping point from normal hemostasis to DVT.
In summary, this study brings up more questions than it answers (as all good studies do). For example, what happens to the risk if there is an intervention to improve calf muscle pump function? Is it possible to modify VTE risk? Would the same results be found in patients with previous DVT or with partial or complete outflow obstruction? Finally, sarcopenia and reduced grip strength have been shown to be important predictors of mortality. Could calf muscle function be used as a clinical adjunct to identify patients requiring prehabilitation prior to surgery? The current study adds to our knowledge and it, along with other ongoing investigations, promises a more personalized approach to VTE prevention and treatment in the future.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal