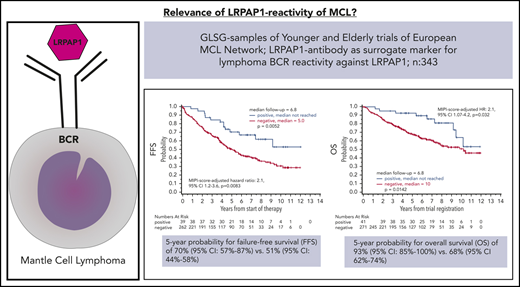
Key Points
LRPAP1-autoantibodies are MIPI-independent markers for superior FFS and OS in MCL treated with immunochemotherapy.
Abstract
Low-density lipoprotein (LDL) receptor-related protein-associated protein 1 (LRPAP1) had been identified by B-cell receptor (BCR) expression cloning and subsequent protein array screening as a frequent and proliferation-inducing autoantigen of mantle cell lymphoma (MCL). Of interest, high-titered and light chain–restricted LRPAP1 autoantibodies were detected in 8 of 28 patients with MCL. In the present study, LRPAP1 autoantibodies in sera of patients treated within the Younger and Elderly trials of the European MCL Network were analyzed regarding frequency, association with disease characteristics, and prognostic impact. LRPAP1 autoantibodies were detected in 41 (13%) of 312 evaluable patients with MCL. These LRPAP1 autoantibodies belonged predominantly to the immunoglobulin G (IgG) class and were clonally light chain restricted (27 with κ light chains, 14 patients with λ light chains). Titers ranged between 1:400 and 1:3200. The presence of LRPAP1 autoantibodies was not significantly associated with any baseline clinical characteristic, however, it was associated with a superior 5-year probability for failure-free survival (FFS) of 70% (95% confidence interval [CI], 57% to 87%) vs 51% (95% CI, 44% to 58%), P = .0052; and for overall survival (OS) of 93% (95% CI, 85% to 100%) vs 68% (95% CI, 62% to 74%), P = .0142. LRPAP1-seropositive patients had a Mantle Cell Lymphoma International Prognostic Index–adjusted hazard ratio for FFS of 0.48 (95% CI 0.27-0.83, P = .0083) and for OS of 0.47 (95% CI 0.24-0.94, P = .032). LRPAP1 autoantibodies were frequently detected in a large cohort of MCL patients treated within prospective multicenter clinical trials. Our results suggest better outcomes for LRPAP1-autoantibody seropositive patients.
Introduction
Mantle cell lymphoma (MCL) accounts for 6% to 8% of non-Hodgkin lymphomas.1 Male and middle-aged to elderly patients are overrepresented, often with advanced stages owing to extralymphatic manifestations.2,3 Immunophenotypically, MCL cells are positive for CD5+, with absent CD23 and CD200. A strong indication for MCL is the overexpression of CyclinD1, because of t(11;14)(q13;q32).4 MCL can be assigned to pregerminal and postgerminal center cells of origin by IgHV mutational or methylation status.5 The frequent occurrence of specific IgHV gene families and interindividually highly similar for instance, stereotypic, CDR3s have been reported as well for MCL.6,-8 This may be seen as a possible indication of shared B-cell receptor (BCR) specificities of MCL subsets against specific target antigens. Fittingly, strong BCR and NF-κB pathway activation was also seen in MCL,9 and antigen-induced activation was rather stronger in MCL compared with other B-cell non-Hodgkin lymphoma.10 Moreover, pharmacological targeting of the BCR pathway by inhibition of PI3K or BTK is an established therapeutic strategy in relapsed/refractory MCL and is investigated for therapy-naive MCL.11,,-14
Low-density lipoprotein (LDL) receptor-related protein-associated protein 1 (LRPAP1) had been identified as such a suspected common autoantigen of BCRs of MCL by our group using BCR expression cloning followed by protein array screening. Thus, 8 of 21 recombinant BCRs from primary MCL cases and the BCRs of the MAVER1 and Z138 lines bound against LRPAP1.15 The gene of LRPAP1 is located at 4p16.3.16 LRPAP1 consists of 357 amino acids and has a molecular weight of 39 kDa, and its main function is reported to be an antagonist and chaperone of the family of LDL receptors and to be involved in Megalin/Cubilin endocytosis.17,18 Immunization of rats with LRPAP1 results in Heymann nephritis.19,20
The pathophysiological background for the immunogenicity of LRPAP1 in MCL has not been elucidated so far. Functionally, in MAVER1 and Z138 MCL cell lines, proliferation could be induced by BCR-pathway stimulation through the addition of LRPAP1. In contrast, the addition of a fusion protein of the epitope of LRPAP1 and a truncated form of exotoxin A of Pseudomonas aeruginosa resulted in apoptosis. Of interest, high-titered and light chain–restricted anti-LRPAP1 serum antibodies were found in 8 of 28 patients of an MCL cohort, but only in 1 of 200 healthy controls.15 Here, we set out to analyze LRPAP1 autoantibodies in sera of patients treated within the prospective, multicenter EU-MCL Younger and Elderly trials in terms of frequency, associations, and impact on clinical outcome.
Methods
Patient and samples
The study had been approved by the local ethics committee (Ärztekammer des Saarlandes 12/13) and was conducted in accordance with the Declaration of Helsinki. Sera were obtained from patients treated in Germany within the European MCL Younger and Elderly trials as part of the minimal residual disease (MRD) program.21,,-24 Previously untreated patients with Ann Arbor stage II to IV MCL had been recruited to the trials and received immunochemotherapy with rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine (oncovin), and prednisone (R-CHOP), alternating R-CHOP/rituximab, dexamethasone, high-dose Ara-C, platinol (cisplatin) (R-DHAP; MCL Younger only; #NCT00209222) or rituximab and fludarabine-cyclophosphamide (R-FC; MCL Elderly only; #NCT00209209). As postremission treatment, high-dose radiochemotherapy followed by autologous stem cell transplantation (MCL Younger) or maintenance with rituximab or interferon-α (MCL Elderly) was applied.23,24
ELISA for LRPAP1-autoantibodies
For enzyme-linked immunosorbent assays (ELISA) of serum autoantibodies against LRPAP1, the expression clone of LRPAP1 spanning from amino acids 192 to 417 (LRPAP1, DKFZp686L1352 from Unipex1) was recombinantly expressed with a C-terminal FLAG tag in HEK293 cells under the control of a cytomegalovirus promoter (pSFI). Total cell extracts were bound at a concentration of 10 µg/mL to Nunc Maxisorb plates precoated overnight at 4°C with murine anti-FLAG antibody at a dilution of 1:2500 (vol/vol; Sigma, Munich, Germany). Blocking was performed with 1.5% (wt/vol) gelatin in Tris-buffered saline (TBS), and washing steps were performed with TBS-Tx (TBS, 0.1% [vol/vol] Tx100). The individual sera were diluted 1:100. ELISAs were conducted according to standard protocols with biotinylated goat anti-human immunoglobulin G (IgG; heavy and light chain), goat anti-human IgA, and anti-human IgM (all Dianova), all at dilutions of 1:2500, or subclass-specific sheep anti-human IgG1, IgG2, IgG3, and IgG4 (Binding Site, Birmingham, UK) at dilutions of 1:5000. After this, corresponding biotinylated secondary antibodies were used for the immunoassays for IgG subclasses. For detection, peroxidase-labeled streptavidin (Roche) was used at a dilution of 1:50 000. Patients were classified as LRPAP1 autoantibody positive if at least 1 serum sample was positive with a titer ≥1:400. Positive samples were subclassified as κ/λ, Ig-type, where applicable IgG subtype and the titer were analyzed. Samples were identified by the trial patient number and the dates for sampling and reception in the Kiel laboratory. Clinical outcome variables were failure-free survival (FFS) and overall survival (OS).
Statistical methods
Clinical characteristics and clinical outcome were compared between patients with and without LRPAP1 data, and subsequently between patients positive and negative for LRPAP1. The analysis of clinical outcome was additionally adjusted for prognostic factors (Mantle Cell Lymphoma International Prognostic Index [MIPI] score).25 Clinical characteristics investigated at diagnosis were study, age, sex, stage, bone marrow involvement, Eastern Cooperative Oncology Group (ECOG) performance status, white blood cell (WBC) count, lactate dehydrogenase level (LDH), MIPI score, MIPI group, Ki-67 index, treatment, and response at end of induction. Outcome variables were OS from trial registration to death from any cause and FFS from treatment start to stable disease, progression, or death from any cause.
As sensitivity analysis, to assess a potential bias for LRPAP1-autoantibody seroconversion occurring at a later time point following treatment start, the prognostic value was investigated in addition according to the first LRPAP1 status assessed restricted to baseline samples (date of reception before or maximally 5 days after start of induction treatment).
Association of MRD status in peripheral blood and pooled peripheral blood and bone marrow at end of induction and after 1 year with LRPAP-autoantibody serostatus were analyzed.
Results
Patients and samples
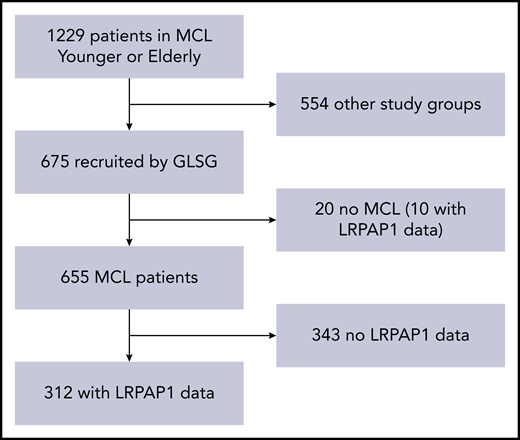
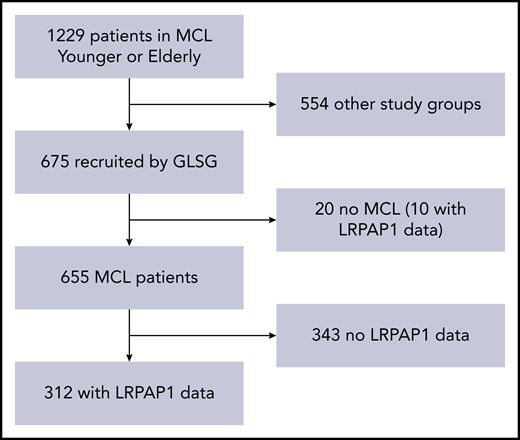
Of 1229 patients included in the EU-MCL Younger and Elderly trials, 675 had been recruited in Germany. Reference pathology verified diagnosis of MCL in 655 of these patients. Serum samples from 312 of these 655 patients could be analyzed for LRPAP1 autoantibodies (Figure 1). Baseline characteristics of these 312 patients are presented in Table 1. These MCL patients with and without serum available for LRPAP1-antibody analysis showed similar characteristics, such as age (median 60 vs 62 years; P = .096), WBC count (median 7.6 vs 7.5 G/L; P = .11), MIPI score (5.78 vs 5.86; P = .21), and Ki-67 index (29% vs 34% with value ≥30%; P = .27), but had more frequently complete or partial response to induction (93% vs 86%; P = .015) and longer FFS (median 5.5 vs 3.2 years; P < .0001) and OS (median 10 vs 5.6 years; P < .0001). The outcome differences can largely be explained by the “immortal time bias”: for the detection of LRPAP1 autoantibodies, not only pretreatment but also follow-up samples were accepted. The samples had been collected primarily for MRD assessment, and patients with MRD samples in follow-up are more likely to be in clinical remission than those without. Thus, when comparing German patients with or without LRPAP1 data at baseline, although the slight differences in age and Ki-67 remained, the percentage of patients with overall response was now similar (89% vs 90%; P = .89; supplemental Table 1, available on the Blood Web site). Furthermore, the outcome differences were substantially smaller (median FFS 5.0 vs 4.0 years, P = .071; median OS 9.5 vs 7.8 years, P = .077).
Patient selection. Of 1229 patients in the MCL Younger and Elderly trials, 675 were recruited by the GLSG. Reference pathology-certified MCL in 655 of these patients. Serum samples from 312 of these 655 patients could be analyzed for LRPAP1 autoantibodies.
Patient selection. Of 1229 patients in the MCL Younger and Elderly trials, 675 were recruited by the GLSG. Reference pathology-certified MCL in 655 of these patients. Serum samples from 312 of these 655 patients could be analyzed for LRPAP1 autoantibodies.
ELISA for LRPAP1-autoantibodies
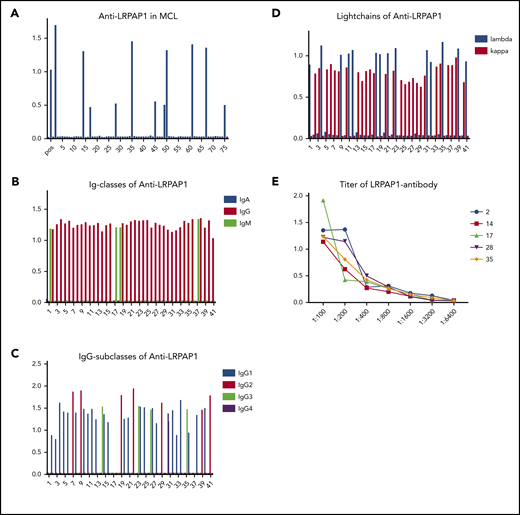
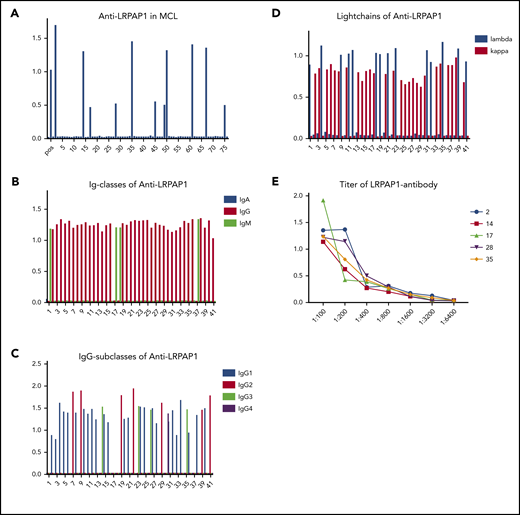
LRPAP1 autoantibodies were detected in 41 of 312 (13%) patients with MCL. These LRPAP1 autoantibodies belonged in 37 patients to the IgG class (IgG1: 25; IgG2: 7; IgG3: 4; and IgG4: 1) and in 4 patients to the IgM class. As expected, LRPAP1 autoantibodies were light chain–restricted, in 27 cases with κ and 14 with λ light chain–restricted autoantibodies. Titers ranged between 1:400 and 1:3200 (Figure 2).
ELISA for LRPAP1 autoantibodies. (A) Representative ELISA of LRPAP1 autoantibodies in 76 sera samples of patients with MCL of whom 11 were positive for LRPAP1 antibodies. The columns represent the optical density measured at 490 nm. In total, in the sera of 41 of 312 patients, MCL autoantibodies directed against LRPAP1 were detected. (B) ELISA of immunoglobulin class of LRPAP1 autoantibodies. LRPAP1 autoantibodies belonged in 37 patients to IgG class and in 4 patients to IgM class. (C) ELISA for IgG subclasses of LRPAP1 autoantibodies: IgG LRPAP1 autoantibodies belonged in 25 cases to IgG1, in 7 cases to IgG2, in 4 cases to IgG3, and in 1 case to IgG4 subclass. (D) ELISA for the light chain of LRPAP1 autoantibodies: LRPAP1 autoantibodies were light chain–restricted (κ: 27; λ: 14). (E) Representative ELISA of titers of LRPAP1 antibodies in seropositive patients with MCL ranged between 1:400 and 1:3200. The curves represent OD measured at 490 nm with sera at indicated dilutions.
ELISA for LRPAP1 autoantibodies. (A) Representative ELISA of LRPAP1 autoantibodies in 76 sera samples of patients with MCL of whom 11 were positive for LRPAP1 antibodies. The columns represent the optical density measured at 490 nm. In total, in the sera of 41 of 312 patients, MCL autoantibodies directed against LRPAP1 were detected. (B) ELISA of immunoglobulin class of LRPAP1 autoantibodies. LRPAP1 autoantibodies belonged in 37 patients to IgG class and in 4 patients to IgM class. (C) ELISA for IgG subclasses of LRPAP1 autoantibodies: IgG LRPAP1 autoantibodies belonged in 25 cases to IgG1, in 7 cases to IgG2, in 4 cases to IgG3, and in 1 case to IgG4 subclass. (D) ELISA for the light chain of LRPAP1 autoantibodies: LRPAP1 autoantibodies were light chain–restricted (κ: 27; λ: 14). (E) Representative ELISA of titers of LRPAP1 antibodies in seropositive patients with MCL ranged between 1:400 and 1:3200. The curves represent OD measured at 490 nm with sera at indicated dilutions.
Out of the 41 patients with seropositivity at any time point, only 4 had a negative baseline sample and converted to positivity after treatment start. In 24 patients, positivity was based on a baseline sample, and 13 did not have a baseline sample.
Of the 312 patients with LRPAP1 data at any time, 86 had 2 or more samples evaluable. The first 2 samples were consistently negative in 70/86, consistently positive in 11/86, 1 positive-negative, and 4 negative-positive. Overall, the concordance rate between the first 2 samples was 94%. In the discordant cases, the time between discordant values was longer than 1 year, so the association to treatment could not be investigated.
Clinical characteristics of seropositive patients
Occurrence of LRPAP1 autoantibodies was not associated with any baseline characteristic (Table 2). A similar percentage of LRPAP1-positive and -negative patients had received the investigational immunochemotherapy in MCL Younger (R-CHOP/R-DHAP: 33% vs 43%; P = .58) and MCL Elderly (R-FC: 64% vs 61%; P = .85).
Prognostic value of LRPAP1 seropositivity
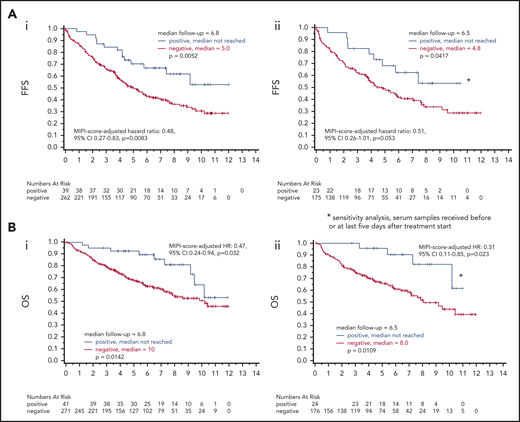
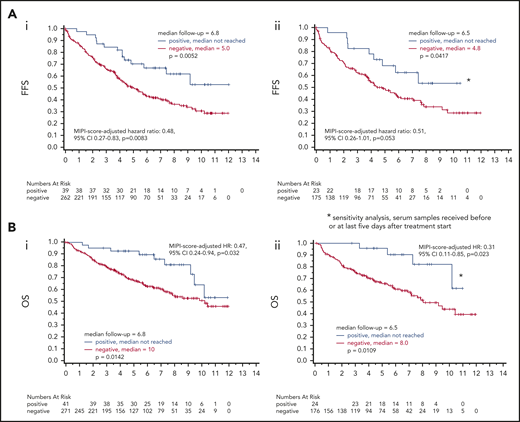
Occurrence of LRPAP1 autoantibodies was associated with a superior FFS (5-year probability 70% vs 51%, log-rank P = .0052; hazard ratio, 0.47; 95% confidence interval [CI], 0.27-0.81) and OS (5-year probability, 93% vs 68%; log-rank P = .0142; hazard ratio, 0.44; 95% CI, 0.22-0.86). These findings were MIPI independent (MIPI score–adjusted hazard ratio for FFS was 0.48; 95% CI, 0.27-0.83; P = .0083; MIPI score–adjusted hazard ratio for OS: 0.47; 95% CI, 0.24-0.94; P = .032). A sensitivity analysis with a more restrictive definition of seropositivity for LRPAP1 autoantibodies (LRPAP1-status assessment restricted to baseline samples with a date of reception maximally 5 days after start of induction treatment) confirmed the association of favorable FFS and OS with positive LRPAP1-autoantibody status (Figure 3).
Prognostic value of LRPAP1 autoantibody. (Ai) After a median follow-up of 6.8 years, for LRPAP1 autoantibody–seropositive patients, the median FFS was not reached. Patients without LRPAP1 autoantibodies had a median FFS of 5 years (P = .0052). The MIPI score–adjusted hazard ratio for FFS was 0.48; 95% CI, 0.27-0.83; P = .0083. (Aii) Using a more restrictive definition of LRPAP1 autoantibody seropositivity by restriction to baseline samples (date of reception before or maximally 5 days after start of treatment), after a median follow-up of 6.5 years, for LRPAP1 autoantibody–seropositive patients, the median FFS was not reached. Patients without LRPAP1 autoantibodies had a median FFS of 4.8 years (P = .0417). MIPI score–adjusted hazard ratio: 0.51; 95% CI, 0.26-1.01; P = .053. (Bi) After a median follow-up of 6.8 years, for LRPAP1 autoantibody–seropositive patients, the median OS was not reached. Patients without LRPAP1 autoantibodies had a median OS of 10 years (P = .0142). MIPI score–adjusted hazard ratio: 0.47; 95% CI, 0.24-0.94; P = .032. (Bii) Sensitivity analysis using a more restrictive definition of LRPAP1 autoantibody seropositivity by restriction to baseline samples (date of reception before or maximally 5 days after start of treatment), after a median follow-up of 6.5 years, for LRPAP1 autoantibody–seropositive patients, the median OS was not reached. Patients without LRPAP1 autoantibodies had a median OS of 8 years (P = .0109). MIPI score–adjusted hazard ratio: 0.31; 95% CI, 0.11-0.85; P = .023.
Prognostic value of LRPAP1 autoantibody. (Ai) After a median follow-up of 6.8 years, for LRPAP1 autoantibody–seropositive patients, the median FFS was not reached. Patients without LRPAP1 autoantibodies had a median FFS of 5 years (P = .0052). The MIPI score–adjusted hazard ratio for FFS was 0.48; 95% CI, 0.27-0.83; P = .0083. (Aii) Using a more restrictive definition of LRPAP1 autoantibody seropositivity by restriction to baseline samples (date of reception before or maximally 5 days after start of treatment), after a median follow-up of 6.5 years, for LRPAP1 autoantibody–seropositive patients, the median FFS was not reached. Patients without LRPAP1 autoantibodies had a median FFS of 4.8 years (P = .0417). MIPI score–adjusted hazard ratio: 0.51; 95% CI, 0.26-1.01; P = .053. (Bi) After a median follow-up of 6.8 years, for LRPAP1 autoantibody–seropositive patients, the median OS was not reached. Patients without LRPAP1 autoantibodies had a median OS of 10 years (P = .0142). MIPI score–adjusted hazard ratio: 0.47; 95% CI, 0.24-0.94; P = .032. (Bii) Sensitivity analysis using a more restrictive definition of LRPAP1 autoantibody seropositivity by restriction to baseline samples (date of reception before or maximally 5 days after start of treatment), after a median follow-up of 6.5 years, for LRPAP1 autoantibody–seropositive patients, the median OS was not reached. Patients without LRPAP1 autoantibodies had a median OS of 8 years (P = .0109). MIPI score–adjusted hazard ratio: 0.31; 95% CI, 0.11-0.85; P = .023.
In the subgroup of 200 patients with both Ki67 data and LRPAP1 data available, the prognostic value of LRPAP1 serostatus was slightly reduced (MIPI score–adjusted hazard ratio for FFS: 0.56; 95% CI, 0.30-1.05; P = .072; MIPI score–adjusted hazard ratio for OS: 0.53; 95% CI, 0.24-1.17; P = .12), because of the smaller number of 200 vs 312 patients. However, additionally adjusting for Ki-67 did not alter the prognostic effects of LRPAP1 serostatus (MIPI score- and Ki-67–adjusted hazard ratio for FFS: 0.59; 95% CI, 0.31-1.11; P = .099; MIPI score- and Ki-67–adjusted hazard ratio for OS: 0.58; 95% CI, 0.27-1.28; P = .18).
LRPAP1-autoantibody serostatus was not significantly associated with MRD status at the end of induction or after 1 year (Table 2).
Discussion
LRPAP1 autoantibodies frequently occur in MCL, and their restriction of light chains and immunoglobulin classes strongly indicates a pathophysiologic relation between serum LRPAP1 autoantibodies and MCL with LRPAP1-reactive BCRs. However, the frequency observed here was lower than previous observations suggested.15 This might be due to the relatively small sample size in the previous study consisting of recombinant. Fabs of 21 patients with MCL, of whom 8 were reactive against LRPAP1, 7 MCL lines of which 2 had LRPAP1-reactive BCRs, and sera of 28 patients with MCL, of whom 8 had LRPAP1 autoantibodies.
An alternative explanation for the discrepancy of LRPAP1 reactivity of recombinant Fabs in the previous study and the frequency of LRPAP1 autoantibodies in this study might be the difference of the applied methods (reactivity screening of recombinant monoclonal Fabs derived from cryospecimen or cell lines vs presence of serum antibodies). Not all MCL patients with LRPAP1-reactive BCRs might develop a humoral immune response with LRPAP1 autoantibodies in the serum.
The presence of LRPAP1 autoantibodies was not associated with any clinical baseline characteristic (including MIPI). Surprisingly, patients with LRPAP1 antibodies showed a better and MIPI-independent FFS and OS when treated with current standard immunochemotherapy, which makes the occurrence of LRPAP1 autoantibodies interesting despite the relatively low observed frequency. The definition of LRPAP1-autoantibody seropositivity can be seen critically. A definition was chosen that patients who were seropositive once during the course of the disease are considered seropositive, which is common in autoimmune diseases. In addition, in a sensitivity analysis with a more restrictive definition of seropositivity before treatment start, the association of LRPAP1 antibodies with an improved outcome was confirmed. Although derived from 2 independent trial cohorts, the prognostic value of LRPAP1 seropositivity in MCL requires independent validation in a future study.
In recent years, prognostic markers for MCL have been identified, such as mutated TP53,26,27 mutated TP53 in combination with Ki67,28 and, more controversially, SOX11.29,30 LRPAP1, in comparison, is a marker that is immunologically detectable in serum/plasma. Adjusting for Ki-67 in addition to MIPI did not relevantly change the prognostic value of LRPAP1 status. Unfortunately, only for a limited number of patients (n = 200), both LRPAP1 data and Ki67 were available, and for this group, even MIPI-adjusted outcome was no longer significant, questioning the statistical power of an adjustment for combined MIPI in this context.
In our previous study, no clear correlation between a specific IgHV sequence or VDJ combination and CDR3 (or stereotype) and the reactivity against LRPAP1 could be found.15 Histologically, MCL cases with LRPAP1-reactive BCRs did not differ from other MCL cases,15 so that detection of LRPAP1 autoantibodies remains an independent factor associated with improved FFS and OS. It would be interesting to investigate the response of the LRPAP1-reactive MCL subgroup to targeted approaches, for example, BTK inhibitors. However, the reason for the better outcome of the LRPAP1-reactive MCL subgroup is unclear. It can be speculated whether these cases are more driven by antigen (LRPAP1)-induced proliferation and thus depend on external stimuli and less intrinsic stimuli (downstream mutations). Moreover, the LRPAP1 reactivity of MCL BCRs might be a therapeutic target for LRPAP1 immunotoxins, LRPAP1-bispecific antibody constructs, or chimeric antigen receptor T cells with LRPAP1 as ectodomain. Even though new therapeutic approaches, such as combined BCL2 and BTK inhibition14 or anti-CD19 chimeric antigen receptor T cells,31 are available and effective for relapsed and refractory MCL, we consider an LRPAP1-specific therapeutic approach because of its high specificity against the malignant clone potentially useful for seropositive patients.
For original data, please contact the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is a Blood Commentary on this article in this issue.
Acknowledgments
The authors are grateful to the entire team of the José Carreras Center for Immuno- and Gene Therapy, the Department of Internal Medicine I of the Saarland University Medical School for continuous logistic and intellectual support, and the German Lymphoma Alliance and former German Low Grade Lymphoma Study Group.
This work was supported by grant 2019.056.1 from Wilhelm Sander-Stiftung (L.T., K.-D.P., and S.H.).
Authorship
Contribution: L.T., J.T.B., K.-D.P., S.H., S.S., M.D., C.P., E.R., E.H., and M.B. designed research, analyzed data, and wrote the paper; N.M., V.P., and D.K.-M. analyzed data; N.F., E.R., R.S., T.R., and O.C. performed experiments; and A.S., H.C.K.-N., O.H., M.D., C.P., and E.H. contributed reagents and clinical data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lorenz Thurner, Department of Internal Medicine I, Saarland University Medical School, D-66421 Homburg/Saar, Germany; e-mail: lorenz.thurner@uks.eu
REFERENCES
Author notes
M.B. and E.H. contributed equally to this study as last authors.