TO THE EDITOR:
Assays that detect leukemia cells at low levels (measurable residual disease [MRD]) by either multiparameter flow cytometry (MFC) or molecular means are increasingly used in caring for patients with acute myeloid leukemia (AML).1 Typically assessed in bone marrow aspirates (BMAs), MRD results have significant prognostic implications.1-5 This has raised interest in using MRD detection to improve prognostic accuracy, risk-stratify patients, detect early relapse, and provide a surrogate biomarker for drug testing. However, the frequency of MRD measurements is limited by the invasiveness of bone marrow aspiration. This limitation could be overcome if MRD assays could be used with similar accuracy in peripheral blood (PB) specimens, and early results with MFC show strong concordance between PB and BMA MFC.6-8 Here, we address PB and BMA MFC concordance further in a large cohort.
Using our AML and hematopathology databases, we identified 724 BMA and PB sample pairs from 477 individual adult patients; samples were considered paired if they were obtained within 1 week of each other. Median patient age was 59 years (range, 19-84 years), and according to the Medical Research Council classification system, 6% had favorable, 61% had intermediate, 25% had adverse, and 8% had unknown cytogenetics.9 Ninety four percent of patients were evaluated between 2008 and 2018 and 6% before 2008 at our center. Three-tube, 10-color MFC with a panel of 3 antibody combinations was performed as previously described.3,5,10 Any level of residual disease was considered MRD-positive, except when otherwise specified.4 During the study period, BMA MFC testing was standard clinical practice; PB MFC testing was at provider discretion and was not standardized or performed at specific clinical time points. Other MRD detection methods (eg, polymerase chain reaction) on either BMA or PB were performed at provider discretion until standardized in February 2015. Complete remission and relapse were defined according to the European LeukemiaNet 2017 classification.11 Multiple PB-BMA sample pairs for an individual patient were analyzed separately. The relationship between PB and BMA blast percentage was measured using Spearman’s rank-order correlation (using the methodology of Rosner and Glynn12 when >1 measurement on patients was included) and by linear regression (mixed models used when >1 measurement on patients was included).13 Statistical analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria).
We first evaluated whether our patient cohort was representative of AML patients at our center, focusing on newly diagnosed (ND) patients. A total of 323 (68%) of 477 patients had ND AML. Of these, 292 arrived at our center between 2008 and 2018, which represented 22% of the 1322 ND AML patients seen during that time period. The complete remission rate among patients with paired samples was 66% vs 51% (P < .0001) for ND AML patients not in our cohort, possibly reflecting a higher proportion of patients younger than age 65 years in our analysis cohort (65% vs 45%; P < .001), although additional selection bias cannot be excluded. The remaining 154 of 477 patients in our cohort were all treated between 2008 and 2018, and they represent 27% of the relapsed/refractory AML patients seen during that period.
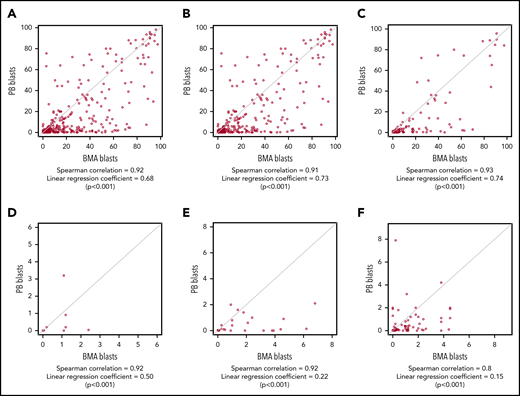
We then examined the relationship between PB and BMA MFC in our cohort of 477 patients regardless of their remission status. Considering all 724 pairs, the Spearman correlation coefficient between PB and BMA blast percentage was 0.92 (Figure 1A) and was 0.91 considering only the first sample pair for each patient (Figure 1B). When considering any level of MRD as positive (MRDpos), 355 of 477 BMA samples were considered MRDpos; of these, paired PB samples were MRDpos in 327 of 355 cases (sensitivity 92%) (Table 1). In BMA, 369 sample pairs were MRD-negative (MRDneg), and 359 of 369 were also MRDneg in PB (specificity 97%) (Table 1). Of the PB MRDpos pairs, 97% were also BMA MRDpos (ie, positive predictive value [PPV]), whereas 93% of PB MRDneg pairs were also BMA MRDneg (ie, negative predictive value [NPV]) (Table 1). Similar results were obtained when considering only a patient’s first pair (Table 1). To address the potential bias arising if PB was examined only after BMA results were known, we also examined the 323 pairs in which PB and BMA were obtained on the same day. Here sensitivity was 91%, specificity was 98%, PPV was 97%, and NPV was 94%, with a Spearman coefficient of 0.93 (Table 1; Figure 1C). We also examined the performance of MFC testing in BMA and PB in pairs within 30 days before hematopoietic stem cell transplant (HCT; n = 32) and within 90 days after HCT (n = 64) (Table 1; Figure 1D-E). Sensitivity (90% and 79%) and NPV (96% and 81%) decreased from before transplant to after transplant, suggesting a higher rate of false-negative PB results in patients immediately post transplant.
Relationship between bone marrow and PB blast percentage measured by MFC in patients with AML. PB and BMA blast percentage measured by MFC are plotted for all sample pairs (A; N = 724), first sample pair for each patient (B; n = 477), pairs for which PB and BMA samples were obtained on the same day (C; n = 323), pairs obtained from patients within 30 days before HCT (D; n = 32), pairs obtained within 90 days after HCT (E; n = 64), and pairs for which BMA blasts were <5% by both flow cytometry and morphology (F; n = 209). Spearman correlation coefficient and linear regression correlation coefficient are shown for each analysis.
Relationship between bone marrow and PB blast percentage measured by MFC in patients with AML. PB and BMA blast percentage measured by MFC are plotted for all sample pairs (A; N = 724), first sample pair for each patient (B; n = 477), pairs for which PB and BMA samples were obtained on the same day (C; n = 323), pairs obtained from patients within 30 days before HCT (D; n = 32), pairs obtained within 90 days after HCT (E; n = 64), and pairs for which BMA blasts were <5% by both flow cytometry and morphology (F; n = 209). Spearman correlation coefficient and linear regression correlation coefficient are shown for each analysis.
Because MRD detection is most relevant at low disease levels, our remaining analyses evaluated pairs in which BMA blast count was <5% by both morphology and MFC (n = 209). The Spearman correlation was 0.8, with sensitivity of 83%, specificity of 95%, PPV of 90%, and NPV of 92% (Table 1; Figure 1F). When using 0.1% abnormal blasts as a cutoff to denote MRDpos samples as proposed by the European LeukemiaNet AML MRD working group,1 sensitivity and NPV of PB were reduced to 62% and 85%, but specificity and PPV remained high (Table 1). We then examined the outcome of patients with discrepant PB-BMA MFC results in this subset, again considering any level of MRD as MRDpos. The 7 PBpos/BMAneg pairs corresponded to 6 patients, and median PB blast percentage was 1.9% (range, 0.01% to 6.9%). Four (67%) of 6 patients developed detectable disease in BMA on a subsequent test in a median of 20 days (range, 7-42 days), and 3 of 4 patients subsequently underwent HCT. The remaining 2 of 6 patients received additional therapy within 1 month of MRD testing and then underwent HCT within 4 to 6 months. The 12 PBneg/BMApos pairs were from 12 different patients. The median BMA blast percentage was 0.17% (range, 0.003% to 3.8%). Four (33%) of 12 patients experienced morphologic relapse in a median 113 days (range, 29-288 days). Six of 8 patients without morphologic relapse underwent treatment to prevent morphologic relapse, with HCT being the next line of therapy in 4 of 6 patients.
We report the largest cohort of paired AML PB and BMA samples pairs analyzed by MFC. In 209 pairs from patients who had disease at the MRD level, PB MFC testing captured 83% of patients with MRD in BMA by MFC with excellent specificity (95%). There was no suggestion that results were biased because PB was obtained only after bone marrow aspiration. Although 0.1% blasts has been proposed as an MFC MRDpos cutoff,1 our data suggest a loss of sensitivity with this threshold, and our previous studies show that any level of abnormal blasts is prognostically informative.3,4 Given this study’s retrospective nature, our cohort included younger patients, PB MFC was not performed at specific times, and there may also be other biases. It is not known whether our findings can be extended to other MRD detection techniques. Furthermore, MFC techniques are not uniform, and our results may not be applicable to other centers with less experience. Given that the sensitivity of PB for BMA disease <90% in some analyses, bone marrow aspiration is still indicated in patients for whom there is high suspicion of relapse. Nonetheless, our demonstration that MRD levels in PB and BMA are highly concordant has potential clinical importance. Although the frequency of MRD monitoring may be limited by cost, PB MFC can be assayed much more frequently than marrow and could replace some BMA studies without dramatic loss of sensitivity and specificity. Thus, PB MFC testing could facilitate serial monitoring, thereby providing AML patients and providers with additional information to guide discussions of prognosis and treatment.
Acknowledgments
This work was supported by a fellowship training grant from the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (T32-HL007093) (C.D.G.) and an institutional K12 grant from the NIH National Cancer Institute (K12-CA076930).
Authorship
Contribution: C.D.G., Y.Z., M.M.A., B.L.W., E.H.E., and R.B.W. designed the study, analyzed data, and performed analyses; M.O., C.M.S., and K.M.G. analyzed data and performed analyses; and all authors contributed to manuscript preparation and revision.
Conflict-of-interest disclosure: C.D.G. received laboratory research grants and/or clinical trial support from Pfizer and Immunogen. R.B.W. received laboratory research grants and/or clinical trial support from Amgen, Aptevo, Celgene, Immunogen, Macrogenics, Jazz, Pfizer, and Selvita; has ownership interests in Amphivena; and is (or has been) a consultant for Agios, Amphivena, Aptevo, Astellas, Bristol Myers Squibb, Celgene, Genentech, Janssen, Kite, Macrogenics, and Pfizer. M.O. is a consultant for Merck and Daiichi Sankyo and is a DSMC member for Celgene and Glycomimetics. The remaining authors declare no competing financial interests.
The current affiliation for Y.Z. is Department of Pathology and Laboratory Medicine, University of Miami, Miami, FL.
Correspondence: Colin D. Godwin, Clinical Research Division, Fred Hutchinson Cancer Research Center; 1100 Fairview Ave N, D2-190, Seattle, WA 98109-1024; e-mail: colindg@uw.edu.
REFERENCES
Contact the corresponding author for original data.