Key Points
Isolated CNS relapse was associated with male sex and BCR-ABL1 fusion in B-ALL and high presenting leukocyte count in T-ALL.
Prephase dexamethasone treatment, delayed initial intrathecal therapy, anesthesia for procedure, and flow cytometry examination of CSF reduced CNS relapse.
Abstract
To identify the prognostic factors that are useful to improve central nervous system (CNS) control in children with acute lymphoblastic leukemia (ALL), we analyzed the outcome of 7640 consecutive patients treated on Chinese Children’s Cancer Group ALL-2015 protocol between 2015 and 2019. This protocol featured prephase dexamethasone treatment before conventional remission induction and subsequent risk-directed therapy, including 16 to 22 triple intrathecal treatments, without prophylactic cranial irradiation. The 5-year event-free survival was 80.3% (95% confidence interval [CI], 78.9-81.7), and overall survival 91.1% (95% CI, 90.1-92.1). The cumulative risk of isolated CNS relapse was 1.9% (95% CI, 1.5-2.3), and any CNS relapse 2.7% (95% CI, 2.2-3.2). The isolated CNS relapse rate was significantly lower in patients with B-cell ALL (B-ALL) than in those with T-cell ALL (T-ALL) (1.6%; 95% CI, 1.2-2.0 vs 4.6%; 95% CI, 2.9-6.3; P < .001). Independent risk factors for isolated CNS relapse included male sex (hazard ratio [HR], 1.8; 95% CI, 1.1-3.0; P = .03), the presence of BCR-ABL1 fusion (HR, 3.8; 95% CI, 2.0-7.3; P < .001) in B-ALL, and presenting leukocyte count ≥50×109/L (HR, 4.3; 95% CI, 1.5-12.2; P = .007) in T-ALL. Significantly lower isolated CNS relapse was associated with the use of total intravenous anesthesia during intrathecal therapy (HR, 0.2; 95% CI, 0.04-0.7; P = .02) and flow cytometry examination of diagnostic cerebrospinal fluid (CSF) (HR, 0.2; 95% CI, 0.06-0.6; P = .006) among patients with B-ALL. Prephase dexamethasone treatment, delayed intrathecal therapy, use of total intravenous anesthesia during intrathecal therapy, and flow cytometry examination of diagnostic CSF may improve CNS control in childhood ALL. This trial was registered with the Chinese Clinical Trial Registry (ChiCTR-IPR-14005706).
Introduction
With 5-year event-free survival and overall survival rates of childhood acute lymphoblastic leukemia (ALL) now exceeding more than 80% and 90%, respectively, in high-income countries,1-11 current efforts focus not only in improving the cure rates but also the quality of life of patients. To reduce neurotoxicity and second malignancy, the most serious late sequelae of childhood ALL survivors, many contemporary protocols have largely or totally omitted the use of prophylactic cranial irradiation and rely on risk-adapted systemic and intrathecal therapy for central nervous system (CNS)-directed treatment.3,5,6,8,10,11 Omission of prophylactic cranial irradiation did not jeopardize leukemia control in the CNS in contemporary risk-directed protocols, with isolated CNS relapse rates ranging from 1.3% to 2.7%,3,5,6,8,10-12 and did not adversely affect the overall survival or the cumulative risk of any relapse in a meta-analysis of data from 10 cooperative study groups.13 The St. Jude Total Therapy Study 16 intensified early intrathecal therapy in patients with the presence of TCF3-PBX1, African ancestry, or any CNS involvement including CNS2, CNS3, or traumatic lumbar puncture with blasts at diagnosis, features associated with increased CNS relapse in the prior Total Therapy Study 15 that totally omitted cranial irradiation.11 However, 1.3% of the patients still experienced isolated CNS relapse in Total Therapy Study 16. Moreover, the increased exposure and duration of total intravenous anesthesia for extra intrathecal treatments may affect neurocognitive function of the patients.14 Hence, research is still necessary to improve CNS control by identifying factors associated with CNS relapse in patients treated in contemporary protocols without the use of cranial irradiation, and to devise measures that can further improve risk-directed therapy, especially for patients treated in countries with limited resources.
We therefore analyzed the data in our recently completed Chinese Children’s Cancer Group ALL-2015 protocol (CCCG-ALL-2015), which totally omitted prophylactic cranial irradiation and included all consecutive patients without exclusion any high-risk subgroups. The treatment components were adopted from Total Therapy Studies 15 and 16,1,11 and modified based on a trial of Shanghai Children’s Medical Center for the drug tolerance of Chinese patients.15 Because of the increased risk of CNS relapse resulting from traumatic lumbar puncture with blasts,16 which necessitates extra intrathecal therapy, and the lack of resources to provide total intravenous anesthesia to optimize the procedure in many participating institutions, we used prephase dexamethasone treatment of 4 to 5 days to reduce leukemia blasts in blood and CNS before the initial intrathecal therapy and conventional remission induction treatment. The findings of this largest clinical trial for childhood ALL reported to date, to our knowledge, suggested some measures that may help to improve CNS control, especially for patients treated in countries with limited resources.
Patients and methods
Participants
Chinese Children’s Cancer Group ALL-2015 is a prospective, multi-institutional clinical trial involving 20 major hospitals/medical centers. Eligible patients were 0 to 18 years old with a confirmed diagnosis of ALL between January 2015 and December 2019. All patients received risk-stratified and minimal residual disease (MRD)-directed treatment. The study was approved by the institutional review board of each participating center, and informed consent was obtained from the parents, guardians, or patients, as appropriate.
Diagnosis and classification
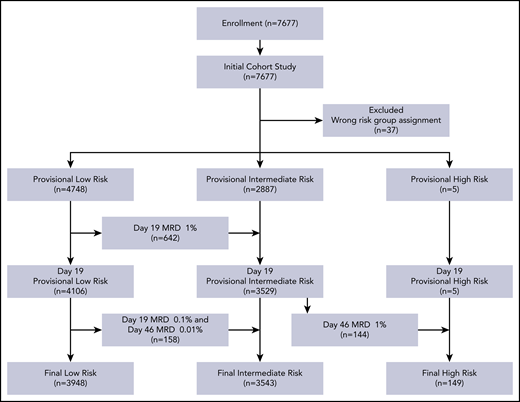
The diagnosis of ALL was based on morphology, immunophenotype, and genetic features of leukemic cells. MRD in bone marrow was measured by flow cytometry. Identification of leukemic cells in cerebrospinal fluid (CSF) was based on conventional cytology, but in 2 centers on flow cytometric analysis, as described previously (see details in supplemental Methods, available on the Blood Web site).17 The low-risk ALL group included patients with B-cell ALL (B-ALL) who were ≥1 and <10 years old and had a white blood cell (WBC) count <50 × 109/L at diagnosis, or had hyperdiploid ≥50 chromosomes or ETV6-RUNX1 fusion; they must not have CNS3 status, testicular leukemia, MRD ≥1% on day 19 of induction, and MRD ≥0.01% on day 46 of induction (supplemental Text box). The high-risk group included all patients with MRD ≥1% or having 5% or more blasts in bone marrow without suitable markers for MRD assay on day 46 of induction, and infants younger than 6 months with KMT2A (MLL) rearrangement and leukocyte count ≥300 × 109/L. The remaining cases were classified as intermediate-risk ALL.
Study design and outcome
The objective of this study was to evaluate the clinical and biological features, treatment, or diagnostic measures that affect CNS control in the context of risk-directed treatment without prophylactic cranial irradiation. The primary end point of this study was isolated CNS relapse. The secondary end points were event-free survival, overall survival, and any CNS relapse. The conduct of the protocol included a central review of MRD, periodic internal and on-site monitoring, and external auditing to ensure protocol compliance and appropriate data management.
Treatment
Prephase treatment with dexamethasone at 6 mg/m2 per day was given for 4 days; patients with presenting leukocyte count ≥50 × 109/L received 1 extra day of dexamethasone at 3 mg/m2 initially to reduce the risk of tumor lysis syndrome (supplemental Table 1). Subsequent remission induction consisted of prednisone, vincristine, daunorubicin, and pegaspargase (days 5-28) followed by cyclophosphamide, mercaptopurine, and cytarabine (days 29-35). Patients with B-ALL who had MRD ≥1% on day 19 of remission induction and all patients with T-cell ALL (T-ALL) received additional early intensification therapy with cyclophosphamide, cytarabine, mercaptopurine, vincristine, and pegaspargase (days 50-57). BCR-ABL1 inhibitor was started after initiation of dexamethasone therapy and continued until the end of therapy for patients with BCR-ABL1–positive ALL. Triple intrathecal therapy was given 3 to 6 times during induction, based on the risk group and CNS status (supplemental Table 1).
Upon completion of induction (between days 46 and 49), consolidation treatment was begun with high-dose methotrexate and triple intrathecal therapy every other week, with daily mercaptopurine for 4 courses (supplemental Table 1). Postremission continuation treatment (weeks 16-53) for patients with low-risk ALL consisted of daily mercaptopurine and weekly methotrexate, interrupted by pulses of dexamethasone plus vincristine with triple intrathecal therapy (every 3-4 weeks) and 2 reinduction treatments. For the patients with intermediate-/high-risk ALL, additional pegaspargase and daunorubicin were given every 3 weeks (supplemental Table 1). Subsequent continuation therapy for patients with low-risk ALL consisted of daily mercaptopurine and weekly methotrexate between weeks 54 and 109, with or without pulse therapy with dexamethasone plus vincristine on week 8. For those in the combined intermediate-/high-risk group, an additional drug pair of cyclophosphamide and cytarabine replaced methotrexate and mercaptopurine every 8 weeks, followed by a rest for 1 week.
Treatment concluded with daily mercaptopurine and weekly methotrexate (weeks 110-125). The low-risk group received 16 to 19 doses of triple intrathecal therapy; the intermediate-/high-risk group received 19 to 22 doses. Neither group received prophylactic cranial irradiation. Allogeneic transplantation or chimeric antigen receptor (CAR) T-cell therapy was a treatment option for patients with high-risk ALL and MRD ≥1% at the end of remission induction.
Statistical analysis
Event-free survival was calculated from the date of diagnosis to the first major adverse event, including induction failure, relapse, death from any cause, development of a second malignant neoplasm, or off-protocol by decision of the treating physician based on severe toxicity. Overall survival was calculated from the date of diagnosis to death from any cause. Time was censored at the date of last patient contact if no event occurred. Event-free survival and overall survival curves were estimated according to the Kaplan-Meier method and compared using the log-rank test. Standard error was estimated by Peto’s method. The cumulative incidence of any relapse or toxicity-related death was estimated by the method of Kalbfleisch and Prentice18 and compared with Gray’s test to account for competing events.19 Competing events for relapse included death during remission, second malignancy, off-protocol by decision of the treating physician, treatment abandonment, and transfer to another hospital. Competing events for death during remission included any relapse, second malignancy, and off-protocol treatment by decision of the treating physician. Confidence intervals (CIs) were calculated with a large-sample normal approximation. Analyses were based primarily on intent to treat. Although the physician’s decision to discontinue treatment was considered as treatment failure, treatment abandonment by parental decision and transfer to another hospital were censored.
Outcome data reported here were updated on 31 July 2020. The median follow-up for the 7173 patients who were alive at the time of analysis was 2.67 years (interquartile range, 1.52-3.85; range, 0.01-5.57); 394 patients had been followed for 5 years or more. Survival and competing risk statistical analyses were conducted with R statistical software version 3.4.4 (The R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/).
Results
Study population
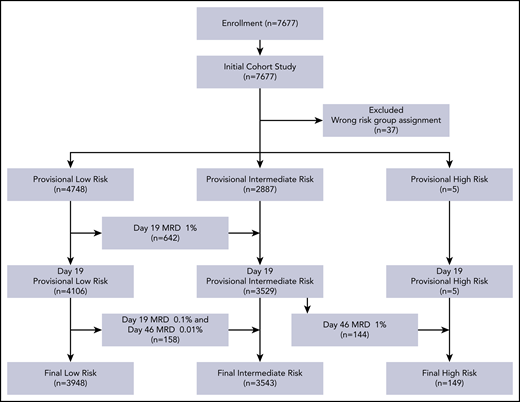
Between 1 January 2015, and 31 December 2019, 7677 consecutive patients with newly diagnosed ALL were enrolled on the protocol. Thirty-seven patients registered in the protocol were excluded from the analyses because they were assigned to the wrong risk group. Table 1 summarizes the characteristics of the 7640 evaluable patients. Their median age at diagnosis was 4.61 years (range, 0.13-17.93), and the median WBC count was 9.66 × 109/L (range, 0.3-936). Based on MRD levels in 7384 patients, we reclassified the risk status of 944 patients: 800 cases were reclassified from provisional low-risk to intermediate-risk, and 144 from provisional intermediate-risk to high-risk (Figure 1).
Treatment outcome
Of the 7640 patients, 7508 (98.30%) entered complete morphologic remission (low-risk, 99.47%; intermediate-risk, 98.33%; and high-risk, 65.10%). Of the 132 induction failures, 73 were due to fatal infections; 50 from refractory leukemia; and 9 from off-protocol by decision of the treating physicians. Among the 149 patients with high-risk ALL, 31 (including 18 with refractory leukemia) underwent allogeneic transplantation 0.4 to 11.6 months (median, 4.2), and 3 received CAR-T therapy 0.8 to 10.9 months (median, 4.6) after remission induction. Of these 34 patients, 20 (including 2 treated with CAR T cells) were alive and in remission for 1.1 to 48.8 months (median, 21.1).
There were 658 relapses: 470 hematologic, 108 isolated CNS, 27 isolated testicular, 26 combined CNS and hematologic, 17 combined hematologic and testicular, 2 combined CNS and testicular, 4 lymph node, 2 ocular, 1 liver, and 1 ovary. Nine patients experienced secondary malignancies: 4 had acute myeloid leukemia; 1 Burkitt lymphoma; 1 intracranial tumor, and 3 Langerhans cell histiocytosis. There were 81 deaths in remission: 65 infections, 3 pancreatitis, 2 transplant-related toxicities, 2 sudden deaths, and 1 each of mechanical intestinal obstruction, cerebral hemorrhage, cardiogenic shock, and hepatic veno-occlusive disease and acute renal failure; 4 causes were unknown.
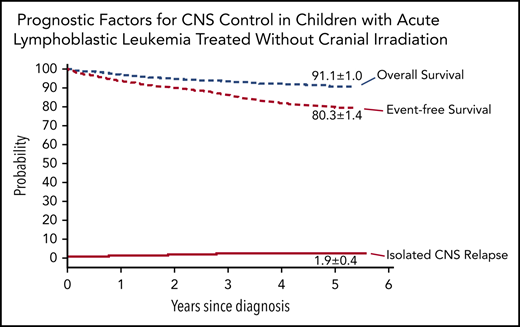
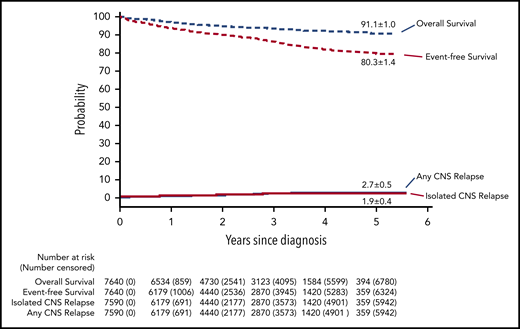
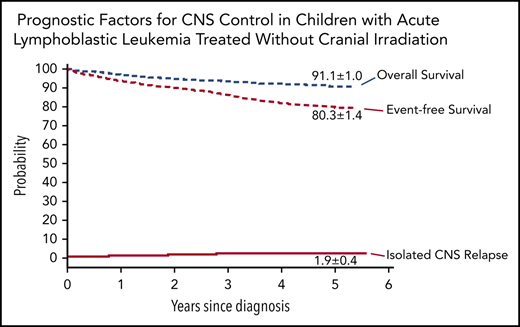
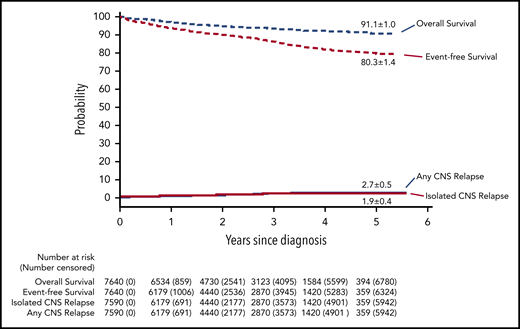
The 5-year cumulative risk of isolated CNS relapse was 1.9% (95% CI, 1.5-2.3) (Figure 2); any CNS relapse, 2.7% (95% CI, 2.2-3.2); isolated hematologic relapse, 10.4% (95% CI, 9.3-11.5); any relapse 15.2% (95% CI, 13.9-16.5); and death during remission, 1.2% (0.9-1.5). The probability of 5-year event-free survival was 80.3% (95% CI, 78.9-81.7), and 5-year overall survival was 91.1% (95% CI, 90.1-92.1).
Outcomes of children with acute lymphoblastic leukemia, as assessed by Kaplan-Meier and Kalbfleisch and Prentice. The 5-year results are shown on the curves.
Outcomes of children with acute lymphoblastic leukemia, as assessed by Kaplan-Meier and Kalbfleisch and Prentice. The 5-year results are shown on the curves.
Impact of clinical and biological factors on event-free survival
Table 1 shows the 5-year event-free survival and overall survival. Because patients with T-ALL had significantly worse outcomes than those with B-ALL, their prognostic factors were analyzed separately (supplemental Table 2). In the multivariate analysis, the independent factors associated with inferior event-free survival of patients with B-ALL were age ≥10 years, male, WBC ≥50 × 109/L, CNS2 or CNS3 status, BCR-ABL1 fusion, KMT2A rearrangements, and the presence of MRD >0.01% at day 19 or 46; those associated with superior outcome were hyperdiploidy >50 chromosomes and the ETV6-RUNX1 fusion (Table 2). Among patients with T-ALL, the significant predictors for lower event-free survival were the BCR-ABL1 fusion and MRD ≥0.1% at day 46.
Impact of clinical and biological factors on CNS relapse
Clinical practice varied among the 20 participating institutions, of which only 3 have resources to provide total intravenous anesthesia (propofol plus fentanyl, ketamine plus midazolam, and both combinations in 1 center each) during lumbar puncture for intrathecal therapy, and 2 have the expertise to examine diagnostic CSF with flow cytometry instead of conventional cytology for the presence of leukemia blasts (supplemental Table 3). The use of total intravenous anesthesia was associated with lower rates of traumatic lumbar puncture (2.1% vs 5.3%, P < .001), whereas the use of flow cytometry was associated with a higher rate of CNS2 status (7.0% vs 0.6%, P < .001; Table 3). There were also substantial differences in the 5-year cumulative risk of isolated CNS relapse (0%-6.6%) and any CNS relapse (0%-15.5%) across the institutions (supplemental Table 3). We therefore included these 2 clinical procedures in the risk factor analyses.
Patients with B-ALL had significantly lower 5-year isolated and any CNS relapse rates than did patients with T-ALL: 1.6% (95% CI, 1.2-2.0) vs 4.6% (95% CI, 2.9-6.3), P < .001; and 2.4% (95% CI, 1.9-2.9) vs 5.7% (95% CI, 3.8-7.6), P < .001. Independent factors associated with increased risk of isolated and any CNS relapse included male sex and the BCR-ABL1 fusion in B-ALL and presenting leukocyte count ≥50 × 109/L in T-ALL (Table 4). Presenting leukocyte count ≥50 × 109/L was associated with increased risk of isolated and any CNS relapse in B-ALL but failed to retain independent significance in multivariate analysis. With intensified intrathecal therapy, CNS2, CNS3, and traumatic lumbar puncture were not associated with isolated or any CNS relapse in B-ALL or T-ALL.
The use of total intravenous anesthesia during intrathecal therapy was independently associated with lower risk of isolated CNS relapse (hazard ratio [HR], 0.2; 95% CI, 0.04-0.7; P = .02) and any CNS relapse (HR, 0.3; 95% CI, 0.1-0.8; P = .02) in B-ALL. The use of flow cytometry to identify leukemia cells in diagnostic CSF was also independently associated with lower risk of isolated CNS relapse (HR, 0.2; 95% CI, 0.06-0.6; P = .006) and any CNS relapse (HR, 0.5; 95% CI, 0.2-0.9; P = .03) in B-ALL.
The isolated and any CNS relapse rates for the 1991 patients treated at the 5 participating institutions with 1 of the 2 services were significantly lower than the rates seen for the 5649 patients treated at the 15 sites without either service: 0.8% (95% CI, 0.3-1.3) vs 2.3% (95% CI, 1.8-2.8; P < .001) and 1.6% (95% CI, 0.9-2.3) vs 3.1% (95% CI, 2.5-3.7; P = .002), respectively. There were no significant differences in 5-year event-free survival or cumulative risk of death during remission between these 2 cohorts: 79.5% (95% CI, 77.0-82.2) vs 80.6% (95% CI, 79.0-82.2; P = .40), and 1.0% (95% CI, 0.5-1.5) vs 1.2% (95% CI, 0.9-1.5; P = .57), respectively. Sixty-four (81.0%) of the 79 patients with B-ALL and 12 (41.4%) of the 29 patients with T-ALL with isolated CNS relapse were alive in second remission for 0.2 to 5.3 years (median, 3.1), and 1.0 to 3.5 years (median, 1.7), respectively.
Treatment toxicity
Except for allergic reaction to asparaginase and thrombosis, patients with intermediate-/high-risk ALL had a higher cumulative risk of grade 4 or 5 adverse events, sepsis, severe pneumonia, invasive fungal infection, pancreatitis, tumor lysis syndrome, seizure, and osteonecrosis than did those with low-risk ALL (Table 5). Patients aged ≥10 years had a higher cumulative risk of grade 4 or 5 adverse events, sepsis, invasive fungal infection, pancreatitis, tumor lysis syndrome, seizure, and osteonecrosis but lower cumulative risk of allergic reaction to pegaspargase (Table 5).
Discussion
The isolated CNS relapse rates of 1.9% (95% CI, 1.5-2.3) and any CNS relapse of 2.7% (95% CI, 2.2-3.2) in this study were comparable to those of other major collaborative study groups (supplemental Table 4), some of which used prophylactic cranial irradiation to treat patients at high risk of CNS relapse.1-12 The numbers of intrathecal therapy (19-22) used in our clinical trial for intermediate-/high-risk patients were also comparable to those (19-26) used in other major collaborative study groups.1-12 Despite limited resources in many of the participating institutions, we attributed our relatively good CNS control to the prephase treatment with dexamethasone, which reduced leukemia cells in blood and the CNS,20,21 and to the delayed intrathecal therapy until all (or a large proportion) of circulating leukemic blasts were cleared, thus reducing the consequence of traumatic lumbar puncture with blasts.22
The proportions of our patients with CNS2 or CNS3 status were lower compared with those in other studies: 1.5% vs 5.3% to 42.1% (median, 11%) and 1.0% vs 1% to 5.7% (median, 2.6%), respectively (supplemental Table 4). Although some of our CNS2 cases might have been missed by conventional cytology examination, 2 other studies that delayed initial intrathecal therapy until after upfront prednisolone or multiagent treatment also reported very low CNS2 status (0% and 0.5%, respectively).5,23 The Tokyo Children’s Cancer Study Group pioneered delayed administration of intrathecal therapy to 1 week after prednisolone treatment in a study of 418 patients.23 In that study, traumatic lumbar puncture occurred in 8.1% of the patients, but CNS2 status was found in only 0.5%. The Taiwan Pediatric Oncology Group conducted a study to delay intrathecal therapy until clearance of blasts in blood for up to 10 days of multiagent remission induction.5 In that study of 152 patients, 2.6% had traumatic lumbar puncture, and none had CNS2 or CNS3 status. This approach of delayed administration of initial intrathecal therapy after prephase steroid treatment, if confirmed successful by additional studies, can be adopted readily by any study groups and would be particularly useful for those in countries with limited resources.
The finding of hyperleukocytosis, BCR-ABL1 fusion, and T-immunophenotype as risk factors for CNS relapse in this study is well recognized.22 Our recent study showed that dasatinib provided better CNS control than did imatinib for BCR-ABL1–positive ALL.24 With additional follow-up of our randomized study, the gap for the difference in isolated CNS relapse rate between dasatinib- and imatinib-treated patients has widened: 3.8% (95% CI, 0-8.1) vs 12.2% (95% CI, 4.9-19.5; P = .05). How can CNS control be improved in T-ALL? Recently, the Children's Oncology Group AALL0434 study showed that nelarabine significantly reduced CNS relapse in patients with T-ALL; however, all nelarabine-treated patients in the study received prophylactic cranial irradiation.25 The current St. Jude Total Therapy study tests if nelarabine can also yield excellent CNS control in patients with T-ALL treated without prophylactic cranial irradiation. Our recent preclinical study showed that dasatinib, a drug with good penetration into the CNS, is effective in 44% of childhood T-ALL,26 a finding suggesting that this drug could also improve CNS control in T-ALL.
This study identified 2 factors that could also improve outcome but would require additional resources: flow cytometry examination of diagnostic CSF and total intravenous anesthesia during intrathecal therapy. Flow cytometric analysis has been shown to have superior sensitivity over conventional cytology to diagnose CNS involvement17 and should facilitate appropriate CNS-directed treatment. Our patients treated in the centers with this capability were more likely found to have CNS2 status and had lower rates of isolated CNS relapse. A recent study also showed that flow cytometric analyses of CSF improves detection of CNS involvement and distinguishes patients with high or low risk of relapse.27 In centers without this expertise, indirect nuclear terminal deoxynucleotidyl transferase immunofluorescence assay, which is more reliable than conventional cytomorphology, can be used.28 The precise identification of CNS2 status is important for intensification of treatment not only to improve CNS control, but also systemic control because CNS2 was independently associated with poorer event-free survival in this and other study.29
The rates of traumatic lumbar puncture, isolated CNS relapse, and any CNS relapse were lower in the patients who had total intravenous anesthesia during intrathecal therapy than in those who did not. It should be cautioned that this finding was not obtained from a randomized trial but was based on a retrospective analysis. However, a historical radioisotope study showed that at least 10% of intrathecal treatments resulted in the drug not being delivered to the subarachnoid space; instead, it was injected or leaked into the subdural or epidural space.30 One would expect an even lower success rate in a frightened child undergoing such a painful procedure without anesthesia. Anesthesia would not only spare some patients from having to endure additional intrathecal treatments for traumatic lumbar puncture at diagnosis, but also optimize intrathecal therapy to improve CNS control. However, anesthesia should be minimized in duration to reduce potential neurocognitive impairment.14
The higher rates of isolated and any CNS relapse in male patients vs female patients in this study were mainly observed among those with B-ALL who did not receive total intravenous anesthesia: 2.2% (95% CI, 1.6-2.8) vs 1.2% (95% CI, 0.7-1.7; P = .02); and 3.3% (95% CI, 2.5-4.1) vs 1.5% (95% CI, 0.9-2.1; P < .001), respectively. One could speculate that it is more difficult to restrict male patients than female patients for successful intrathecal therapy if they were not undergoing anesthesia during the procedure.
CNS2, CNS3, and traumatic lumbar puncture were not associated with the risk of CNS relapse in this study, probably because patients with these statuses received more intensive intrathecal therapy, as was also observed in the Total Therapy 16 study.11 Notably, CNS3 status was associated with poorer event-free survival, but it had no impact on the risk of CNS relapse; only 1 of 55 patients with B-ALL and 1 of 21 with T-ALL with CNS3 status experienced isolated CNS relapse.
The treatment of this clinical trial was relatively well tolerated, with only a 1.2% cumulative risk of death during remission. There were no excessive rates of other major side effects. The very low rate (0.4%) of osteonecrosis in this study is consistent with the experience in a recent study in Japan,31 suggesting that East Asians may be at lower risk of this complication. Notwithstanding these findings, further improvement of clinical outcome in children with ALL relies on the incorporation of novel molecular therapeutics and immunotherapy without overlapping toxicities into clinical trials.32,33 Finally, it will be of interest to study neurocognitive function of our survivors having received intensive intrathecal, high-dose methotrexate, and dexamethasone treatment without cranial irradiation.
Deidentified patient-level data including presenting features, treatment response, and follow-up, along with the study protocol, are available by contacting Jingyan Tang (tanglingyan@scmc.com.cn) or Jiaoyang Cai (caijiaoyang@scmc.com.cn).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is a Blood Commentary on this article in this issue.
Acknowledgments
This study was supported by grants from the VIVA China Children’s Cancer Foundation, the National Natural Science Foundation of China (81670136) (J.T. and J.C.), the fourth round of Three-Year Public Health Action Plan (2015-2017) (GWIV-25) (S.S.), National Institutes of Health, National Cancer Institute (CA21765) (C.C., J.J.Y., and C.-H.P.), St. Baldrick’s Foundation (581580) (H.J.), and the American Lebanese and Syrian Associated Charities (C.C., J.J.Y., and C.-H.P.).
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health.
Authorship
Contribution: C.-H.P., J.T., J.Y., C.-k.L., X.Z., and S.S. conceived and performed the research, discussed data, and wrote the paper; J.C. and C.C. provided statistical analysis; and all authors contributed to the manuscript and interpretation of the data and approved the final version of the manuscript.
Conflict-of-interest: The authors declare no competing financial interests.
Correspondence: Ching-Hon Pui, Department of Oncology, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105-3678; e-mail: ching-hon.pui@stjude.org.
REFERENCES
Author notes
J.T., J.Y., J.C., L.Z., and S.H. contributed equally to this article as first authors.
C.-k.L., X.Z., S.S., and C.-H.P. contributed equally to this article as senior authors.