Abstract
Introduction: The poor prognosis of acute myeloid leukemia (AML) and the highly heterogenous nature of the disease
motivates targeted gene therapeutic investigations. Rho-associated protein kinases (ROCKs) are crucial for various
actin cytoskeletal changes, which have established malignant consequences in various cancers, yet are still not being
successfully utilized clinically towards cancer treatment. ROCK 1 and 2 overexpression has been linked to AML cell
lines and overall survival of AML patients. This work reports the considerable therapeutic efficacy of the ROCK
inhibitor DJ4 in both in vitro and in vivo preclinical models of AML to highlight the potential of this class of inhibitors.
Experimental Design: The cytotoxic and pro-apoptotic activities of DJ4 for primary human AML samples and human
AML cell lines was determined by cell viability (MTS), colony forming assays, and Annexin V assays. Immunoblot
analysis was used to detect phosphorylation of downstream substrates of ROCK. To assess the preclinical therapeutic
efficacy, the luciferase-expressing human AML cell line OCIAML-3-YFP-Luc was injected subcutaneously (SC) and
intravenously (IV) into NRG-S mice. Mice were treated through an intraperitoneal (IP) injection with either vehicle
control or DJ4 (10 mg/kg) for 3 weeks. Modified AML cell lines, OCI-AML3-YFP-Luc and MV4-11-Luc2-EGFP, were
also treated with their respective IC50 dose of DJ4 or with vehicle control for 24 h and then administered via IV into
NRG-S mice and the survival advantage was monitored over time without further treatment. Disease progression was
tracked in mice studies by flow cytometry analysis and examining the survival, bioluminescent signal, tumor volume,
and tumor weights of the animals over time.
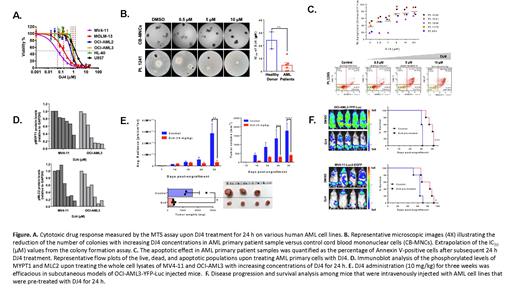
Results: DJ4 induced cytotoxic and pro-apoptotic effects, within the micromolar range and in a dose-dependent
manner, in human AML cell lines (IC50: 0.05-1.68 μM) and primary patient cells (IC50: 0.264-13.43 μM) that harbored
various mutations; however, normal hematopoietic cells are largely spared (Figures A-C). Treatment of DJ4
demonstrated ~5-fold selectivity towards AML patient samples relative to the CB-MNCs of healthy donors (IC50= 25
μM, Figure B). Representative flow cytometry plots of the Annexin V assay with AML primary cells (Figure C) depicted
the increase in the apoptotic populations as a result of treatment with increasing concentrations of DJ4. ROCK
inhibition by DJ4 disrupts the phosphorylation of downstream targets, myosin light chain (MLC2), and myosin binding
subunit of MLC phosphatase (MYPT) in OCI-AML3 and MV4-11, yielding a potent yet selective treatment response at
micromolar concentrations, 0.02 to 1 μM (Figure D). Analysis of mice treated with DJ4 via IP for 2.5 weeks indicated
that DJ4 was well-tolerated with comparable CBC with differential and chemistry values and tissue morphology from
cross-sections of the spleen, liver, lung, and kidney relative to the vehicle treated group. Mice that were IV or SC
injected with OCIAML3-YFP-Luc and treated via IP with DJ4 exhibited an increase in overall survival and reduction
in disease progression relative to the vehicle treated control mice (Figure E). Towards the end of the study, the
OCIAML3-YFP-Luc SC injected mice exhibited at least a 3-fold reduction in the bioluminescent signal, ~4-fold decrease
in tumor volume, and the tumor weight was significantly reduced by ~3-fold among the DJ4 treated group relative to
the control (Figure E). Mice that were IV administered DJ4 pretreated AML cells also exhibited a decreased
bioluminescent signal, increased survival of 10 and 20 days, and a significant reduction in the percent of human CD45
cells in the bone marrow or spleen, indicating a diminished tumor burden (Figure F).
Conclusion:
The observed potency of DJ4 towards various AML cell lines and primary samples with a diverse set of mutations
suggests that this is a promising candidate to be incorporated into the standard AML regimen to help many different
subsets of AML patients. Therapeutics that induce apoptosis have been shown to be promising candidates toward
overcoming chemoresistance and to work well with appropriate combinations of standard of care drugs. This work
highlights the potential of targeting the Rho-ROCK pathway to improve the prognosis of AML and the need for the
future development of chemotherapeutics that are both less toxic and more effective.
Claxton: Astellas: Other: Clinical Trial; Novartis: Research Funding; Astex: Research Funding; Cyclacel: Research Funding; Daiichi Sankyo: Research Funding; Incyte: Research Funding.