TO THE EDITOR:
Despite vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there is increased risk of breakthrough infections among persons with immune dysfunction such as those with autoimmune disease on immunosuppression, and individuals with chronic inflammation, B-cell lymphoma, or solid organ transplants.1 Breakthrough infections are of particular concern among patients with hematologic malignancy who have been demonstrated to fare particularly poorly when infected with SARS-CoV-2.2
Myeloid neoplasms such as myelodysplastic syndromes (MDS), acute myeloid leukemia (AML), and myeloproliferative neoplasms are often observed in older persons, and can share similar disease ontogeny. Infectious complications are a key contributor to mortality for patients with these conditions.3-7 Patients with myeloid neoplasms are underrepresented in studies of response to SARS-CoV-2 vaccines.3,8-10 Although prioritized for early vaccination, to our knowledge, the ability of patients with myeloid neoplasms such as MDS to elicit neutralizing antibodies against the ancestral strain and variants of concern (VOCs) has not been reported to date.
We evaluated a cohort of patients with myeloid neoplasms (n = 48; median age, 70 years; range, 28-89 years) receiving standard therapies (eg, supportive care, growth factors, DNA hypomethylating agents, or kinase inhibitors) for their neutralizing antibody responses to vaccine-homologous SARS-CoV-2 WA1/2020 strain and 5 VOCs in the periods following second and third (booster) vaccinations (Table 1). Two patients had documented breakthrough SARS-CoV-2 infection after 2 vaccine doses, with 1 patient (P-8) having prolonged hospitalization but ultimately recovered, and a second patient (P-30) with mild symptoms and was managed as an outpatient (supplemental Table 1). After the third vaccine dose, 1 patient (P-53) had documented breakthrough SARS-CoV-2 infection resulting in hospitalization and recovered fully. Supplemental Table 1 lists the extended clinical characteristics, treatment summary, vaccine/booster type, and the time point of sampling relative to coronavirus disease 2019 (COVID-19) vaccination. Healthy health care workers (n = 16) working at a research institution and who were neither exposed to SARS-CoV-2 and do not work with patients with COVID-19 were used as the comparative control cohort (median age, 34.5 years; range, 21-75 years). None of the healthy controls had breakthrough SARS-CoV-2 infections.
We performed SARS-CoV-2 virus neutralization assays, which in contrast to conventional assays that measure SARS-CoV-2 binding antibodies, can distinguish the capacity of immune sera to block cell entry by the prototype WA1/2020 strain, used in the vaccines, as well as individual VOCs. Virus neutralization titers have been correlated with protection against SARS-CoV-2 infections and especially against severe disease. Postvaccination sera were evaluated in a qualified SARS-CoV-2 pseudovirion neutralization assay (PsVNA) using SARS-CoV-2 WA1/2020 strain and the 5 VOC strains: Alpha, Beta, Gamma, Delta, and Omicron (supplemental Methods). SARS-CoV-2 neutralizing activity measured by PsVNA as 50% neutralization titers (PsVNA50) correlated with plaque reduction neutralization test with authentic SARS-CoV-2 virus in our previous studies.11 The median time intervals between vaccination and evaluation of sera in MDS/AML patients were 150.5 days following the second vaccination (n = 38) and 30.5 days following the third vaccination (n = 11), whereas for healthy controls, it was 38 days after the second vaccination (n = 16) and 57.5 days after the third vaccination (n = 16) (Table 1).
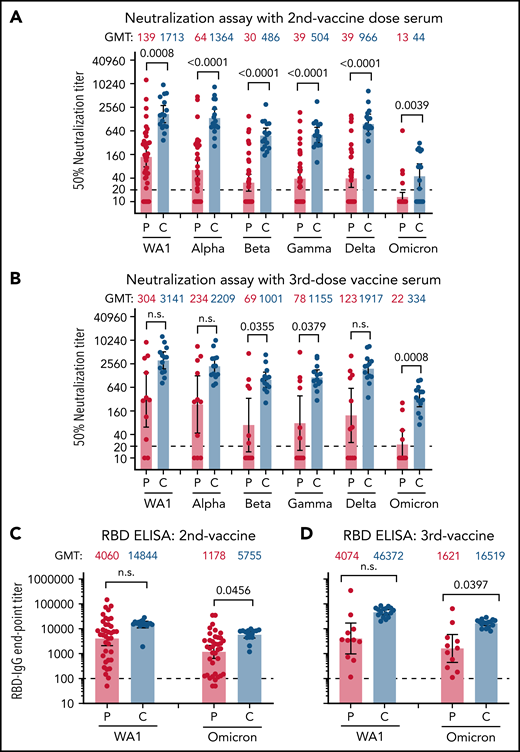
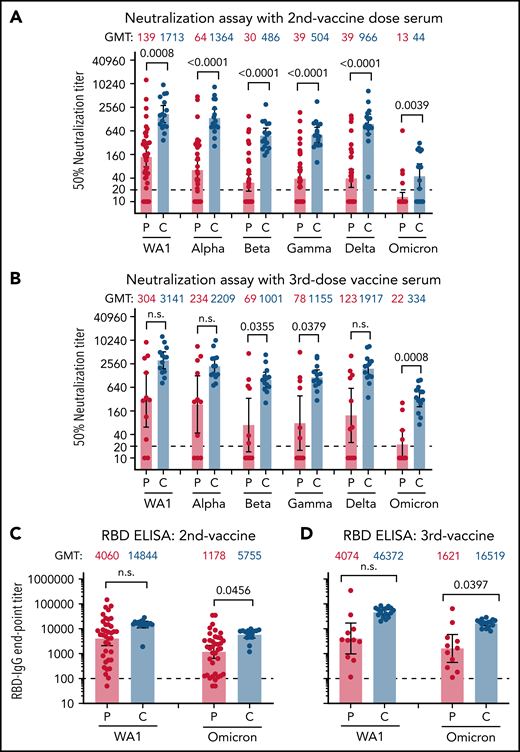
After 2 vaccinations, the control group demonstrated a robust response (100% with PsVNA50 >1:40) against the vaccine homologous WA1/2020 (PsVNA50 geometric mean titer [GMT] of 1:1713). In contrast, patients with myeloid neoplasms (n = 38) displayed significantly weaker neutralization titers with GMT of 1:139 (7/38 nonresponders with PsVNA50 of <1:20) (Figure 1A; supplemental Table 2). We did not observe significant differences in vaccine response between patients with AML (n = 9), MDS (n = 17), or myeloproliferative neoplasms (n = 12) after 2 vaccine doses.
Neutralization and antibody binding of postvaccination serum against SARS-CoV-2 WA1/2020 strain and variants of concern. Neutralization assays were performed with the use of pseudoviruses expressing the SARS-CoV-2 spike proteins of the WA1/2020 vaccine strain or the Alpha, Beta, Gamma, Delta, and Omicron variants. (A) Serum samples following 2 doses of SARS-CoV-2 mRNA vaccination were obtained from 38 patients with AML/MDS (P; in red) or 16 healthy controls (C; in blue). (B) Vaccination samples from after the third vaccination were obtained from 11 patients with AML/MDS and 16 healthy controls. The heights of the bars and the numbers over the bars indicate the GMTs; the whiskers indicate 95% confidence intervals and are color-coded. The assay of each serum sample was performed in duplicate. Each data point represents an individual sample (circles) and indicates the 50% neutralization titer obtained with each sample against the corresponding pseudovirus. The horizontal dashed line indicates the limit of detection for the neutralization assay (PsVNA50 of 20). The raw data and information regarding the serum samples from vaccinated participant (sex, age, vaccine type, and samples collected postvaccination and 50% neutralization titers against various SARS-CoV-2 strains) are summarized in supplemental Table 2. Differences between SARS-CoV-2 strains were analyzed by lme4 and emmeans packages in R using Tukey pairwise multiple comparison test and the P values are shown. (C,D) SARS-CoV-2 RBD-binding IgG to vaccine-homologous WA1/2020 and Omicron variant in serum samples following 2 doses of SARS-CoV-2 mRNA vaccination (C) from 38 patients with AML/MDS (P; in red) and 16 healthy controls (C; in blue) or following 3 doses of vaccination (D) from 11 patients with AML/MDS (P; in red) and 16 healthy controls (C; in blue). Each serum sample was evaluated in IgG-ELISA in duplicate to determine the RBD-binding IgG end-point titer against RBD of either WA1/2020 or the Omicron variant. The height of bars and numbers over the bars indicate the IgG GMTs, and the whiskers indicate 95% confidence intervals. The horizontal dashed line indicates the limit of detection for IgG ELISA (1:100). Statistical differences between patients and controls were analyzed by lme4 and emmeans packages in R using Tukey pairwise multiple comparison test and the P values are shown. ELISA, enzyme-linked immunosorbent assay.
Neutralization and antibody binding of postvaccination serum against SARS-CoV-2 WA1/2020 strain and variants of concern. Neutralization assays were performed with the use of pseudoviruses expressing the SARS-CoV-2 spike proteins of the WA1/2020 vaccine strain or the Alpha, Beta, Gamma, Delta, and Omicron variants. (A) Serum samples following 2 doses of SARS-CoV-2 mRNA vaccination were obtained from 38 patients with AML/MDS (P; in red) or 16 healthy controls (C; in blue). (B) Vaccination samples from after the third vaccination were obtained from 11 patients with AML/MDS and 16 healthy controls. The heights of the bars and the numbers over the bars indicate the GMTs; the whiskers indicate 95% confidence intervals and are color-coded. The assay of each serum sample was performed in duplicate. Each data point represents an individual sample (circles) and indicates the 50% neutralization titer obtained with each sample against the corresponding pseudovirus. The horizontal dashed line indicates the limit of detection for the neutralization assay (PsVNA50 of 20). The raw data and information regarding the serum samples from vaccinated participant (sex, age, vaccine type, and samples collected postvaccination and 50% neutralization titers against various SARS-CoV-2 strains) are summarized in supplemental Table 2. Differences between SARS-CoV-2 strains were analyzed by lme4 and emmeans packages in R using Tukey pairwise multiple comparison test and the P values are shown. (C,D) SARS-CoV-2 RBD-binding IgG to vaccine-homologous WA1/2020 and Omicron variant in serum samples following 2 doses of SARS-CoV-2 mRNA vaccination (C) from 38 patients with AML/MDS (P; in red) and 16 healthy controls (C; in blue) or following 3 doses of vaccination (D) from 11 patients with AML/MDS (P; in red) and 16 healthy controls (C; in blue). Each serum sample was evaluated in IgG-ELISA in duplicate to determine the RBD-binding IgG end-point titer against RBD of either WA1/2020 or the Omicron variant. The height of bars and numbers over the bars indicate the IgG GMTs, and the whiskers indicate 95% confidence intervals. The horizontal dashed line indicates the limit of detection for IgG ELISA (1:100). Statistical differences between patients and controls were analyzed by lme4 and emmeans packages in R using Tukey pairwise multiple comparison test and the P values are shown. ELISA, enzyme-linked immunosorbent assay.
Booster (third) vaccination in the healthy controls resulted in consistently strong neutralizing antibody responses against the WA1/2020 strain (PsVNA50 >1:500; GMT, 1:3141). In contrast, among 11 patients with myeloid neoplasms (diagnosed as MDS or AML) who received 3 vaccine doses, WA1/2020 neutralizing antibodies were highly variable (GMT, 1:304), with 2/11 demonstrating no neutralization response (PsVNA50 <1:20), and only 4/11 strong responders (PsVNA50 >1:500) against WA1/2020 (Figure 1B; supplemental Table 2).
In healthy adults, 2 vaccinations demonstrated 1.3-, 3.5-, 3.4-, and 1.8-fold reduction against Alpha, Beta, Gamma, and Delta variants, respectively, and more pronounced loss of activity (38.9-fold) against Omicron, compared with the vaccine-homologous WA1/2020 (Figure 1A; supplemental Table 2). Following a third vaccine dose, neutralization titers in the healthy cohort increased modestly against Alpha (1.7-fold), Beta (2.1-fold), Gamma (2.3-fold), and Delta (2.0-fold) variants compared with the second vaccination. Moreover, the third vaccination improved neutralization titer against Omicron (GMT, 1:334) by 7.6-fold compared with antibody response following the second vaccination (GMT, 1:44); but still the neutralizing antibodies‘ GMT against Omicron was reduced by 9.4-fold relative to WA1/2020 (Figure 1B; supplemental Table 2).
In contrast to healthy controls, the majority of patients with myeloid neoplasms demonstrated minimal or no neutralizing antibodies against the VOCs including Omicron (92% patients with PsVNA50 <1:20 against Omicron) after 2 vaccinations (Figure 1A; supplemental Table 2), except for patient P-19 (an 82-year-old woman with MDS). Among the patients who received 3 vaccinations, the majority (7/11) exhibited much lower neutralization responses against all VOCs and no neutralization titers against Omicron compared with healthy controls (Figure 1B). Even the 4 patients that exhibited strong anti-WA1/2020 responses (P-52, P-54, P-55, and P-30R; PsVNA50 >1:1000) demonstrated profoundly lower responses against Omicron (PsVNA50 of 1:52, 1:30, 1:169, and 1:257, respectively) after the third vaccination (supplemental Table 2). The patient (P-30R) with the highest neutralization titer (1:257) against Omicron after the third vaccination had a breakthrough SARS-CoV-2 infection after the second vaccination.
The low neutralization titers observed in MDS/AML against both WA1/2020 and VOCs did not provide a complete picture regarding their antibody response to vaccination. Therefore, we also measured IgG binding to the SARS-CoV-2 spike receptor-binding domain (RBD) derived from vaccine-homologous WA1/2020 as well as Omicron variant using enzyme-linked immunosorbent assay (Figure 1C-D). Binding to both WA1 and Omicron RBD was robust for the healthy adults after 2 and 3 vaccinations (Figure 1C-D). In comparison, after the second vaccination, the RBD-binding antibodies from patients with MDS/AML were more variable, with 11 of 38 patients demonstrating RBD-IgG end-point titers below 1:200 serum dilution against the Omicron RBD and lower than the healthy cohort (Figure 1C). Patients with MDS/AML receiving 3 vaccinations showed increased antibody binding titers against the Omicron RBD, but they were still lower than the healthy adults (Figure 1D). A correlation was observed between SARS-CoV-2 RBD-binding antibody titers and SARS-CoV-2 neutralization titers for these 2 cohorts that received either 2 or 3 vaccinations (supplemental Figure 1).
Large-scale vaccine effectiveness studies evaluating clinical outcomes and complications of COVID-19 infections demonstrated slightly lower effectiveness in persons with coexisting conditions.12 The correlates of protection for SARS-CoV-2 strains before circulation of antibody-resistant Omicron variant suggested neutralizing antibody titers above 1:60 can reasonably provide protection against severe COVID-19.13,14 Based on these studies, the healthy individuals who received 3 doses of the messenger RNA (mRNA) vaccines are likely to be protected against severe disease from the Omicron variant. It is possible other components of immune system in addition to neutralizing antibodies including T cells can contribute to protection from severe disease. However, the effectiveness of 2 doses of mRNA vaccine in patients with hematological neoplasms was found to be significantly reduced after mass vaccination in Israel, showing 1.7 to 2.3 increased risk of symptomatic disease, hospitalization, and death.15
Patients with myeloid neoplasms, especially those with high-risk diseases such as MDS and AML who are often treated with similar hypomethylating agent-based chemotherapy regimens, are underrepresented in the published literature reporting the efficacy of SARS-CoV-2 vaccination. Our findings demonstrate that mRNA vaccination in a cohort of patients with myeloid neoplasms, both untreated and receiving standard of care therapeutics, elicited variable titers of RBD-binding antibodies. But most of these patients had low (or none) neutralizing antibody responses against the Omicron variant following 2 or 3 COVID-19 vaccinations that should be confirmed in larger studies. A recent study by Mori et al reported similar seroconversion rates in healthy controls and patients with MDS/AML, especially those not on treatment, after mRNA vaccination.16 However, only anti-spike binding antibodies were measured in the study. Our study underscores the importance of measuring virus neutralization titers, both against the vaccine strain and against clinically relevant circulating VOCs such as Omicron.
These observations highlight the immunodeficiency in this patient population. Even patients with MDS on observation generated weak neutralizing antibody response following SARS-CoV-2 vaccination. These patients are likely to be at increased risk for breakthrough infection, especially from Omicron, and therefore should be prioritized for postexposure treatments early after SARS-CoV-2 infection.
Acknowledgments
The authors thank Basil Golding and Keith Peden at the Food and Drug Administration (FDA), and Philip McCarthy at Roswell Park Comprehensive Cancer Center for review of the manuscript. They also thank Carol Weiss (FDA) and National Institutes of Health Vaccine Research Center for providing plasmid clones expressing SARS-CoV-2 spike variants.
This study was funded by grants from the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism (NIAID) Inter Agency Agreement (IAA; AA121031) and the National Cancer Institute (P30CA016056) using Roswell Park Comprehensive Cancer Center’s Hematologic Procurement Shared Resource. Antibody response study was supported by the FDA (MCMi OCET 2021-1565 to S.K.), NIAID (IAA AA121031), donations from the family of Kathleen Wieczkowski and Robert Drajem, and the Fighting Irish Fighting Cancer Team via the Roswell Park Alliance Foundation (E.A.G.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Authorship
Contribution: S.K., H.G., and E.A.G. designed the research; P.S., M.J.N., and E.A.G. collected clinical specimens and unblinded the clinical data; L.B., G.G., and S.K. performed assays; and S.K., H.G., M.J.N., and E.A.G. contributed to writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Surender Khurana, Division of Viral Products, Center for Biologics Evaluation and Research (CBER), Food and Drug Administration (FDA), 10903 New Hampshire Ave, Silver Spring, MD 20993; e-mail: surender.khurana@fda.hhs.gov.
All data needed to evaluate the conclusions in the article are present in the manuscript.
The online version of this article contains a data supplement.