TO THE EDITOR:
Anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma (ALK-LBCL) is a rare lymphoma characterized by morphology and immunophenotype of plasmablasts and driven by oncogenic ALK fusions.1-3 The most frequent abnormality is t(2;17)(p23;q23) fusing ALK with the clathrin heavy-chain gene (CLTC), although other fusions have been described.1,4-9 ALK-LBCL is associated with a dismal prognosis, reflecting its chemoimmunotherapy refractoriness and absence of effective targets.2 Its poor prognosis is pronounced in the relapsed/refractory setting where, to our knowledge, no long-term survivors have been reported.
CLTC-ALK fusion protein drives constitutive ALK activity on which ALK-LBCL appears dependent.10 ALK inhibitors (ALKi) have shown efficacy in ALK-rearranged anaplastic large cell lymphoma and non-small-cell lung cancer.11-15 Anecdotal reports of the first-generation ALKi crizotinib with cytotoxic chemotherapy in ALK-LBCL have been discouraging.16
Alectinib and lorlatinib are next-generation ALK inhibitors with higher potency than crizotinib and are effective in crizotinib-refractory ALK-positive cancers.11-13 We generated the first patient-derived xenograft (PDX) models of ALK-LBCL and evaluated the therapeutic activity of a higher potency ALKi. Based on promising efficacy in these models, we tested alectinib in 4 consecutive patients with relapsed/refractory ALK-LBCL.
Portions of tumor biopsies from 2 patients with relapsed/refractory ALK-LBCL were obtained with informed consent and xenografted under Dana-Farber/Harvard Cancer Center institutional review board protocol #13-351. Tumor seeds were engrafted by renal capsule implantation (RCI) into Nod.Cg-PrkdcSCIDIL2Rγ<tm1Wjl>/SzJ (NSG) mice (Jackson Laboratories). Engrafted tumors were repassaged and both subcutaneous implantation (SCI) and RCI PDXs were generated. Mice were handled per Dana-Farber/Harvard Cancer Center Institutional Animal Care and Use Committee-approved protocol #13-034.
RCI and SCI engrafted mice were monitored with ultrasound and caliper measurements respectively. Upon engraftment, mice were assigned to tumor volume-matched groups and dosed daily with lorlatinib, alectinib, crizotinib, or vehicle (acidified water) by oral gavage. Tumor volume was measured daily thereafter.
We identified 4 consecutive patients with relapsed/refractory ALK-LBCL. Alectinib was administered at 600 mg PO twice daily off-label.2 Responses were assessed per Lugano Criteria.17 All patients provided written informed consent.
A fresh tumor biopsy was taken from a patient with ALK-LBCL (Figure 1A-E) refractory to 8 therapies including progressive disease on crizotinib (supplemental Material available on the Blood Web site).
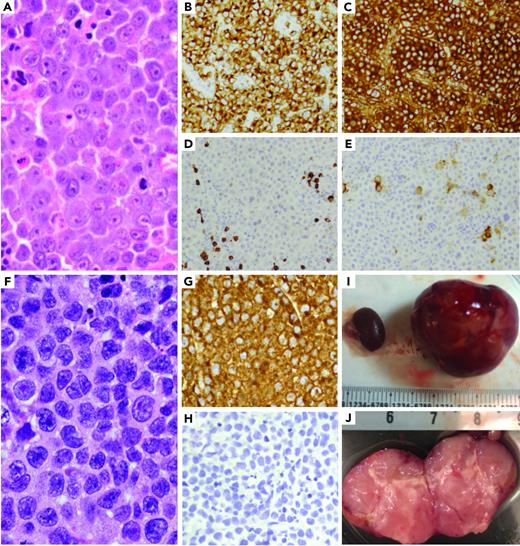
ALK-positive large B-cell lymphoma patient-derived xenografts. (A-E) ALK-positive large B-cell lymphoma morphology shows sheets of large neoplastic lymphoid cells with plasmablastic morphology (A), including moderate to abundant cytoplasm and round to irregular nuclei with prominent nucleoli. The neoplastic cells are positive ALK1 (B) and IgA (C), and negative for CD20 (D) and CD30 (E). (F-J) A PDX model of the ALK-positive large B-cell tumor was generated by implanting a tumor fragment underneath the kidney capsule, which generated a tumor mass after 6 to 8 weeks. The morphology of the PDX model (F) was identical to that of the patient's tumor, with strong ALK1 expression (G) and negative for CD20 (H). (I) PDX tumor replacing the implanted left kidney of mouse (right) and uninvolved right kidney on left (millimeters). (J) PDX bivalved showing tan, soft-cut surface of tumor (centimeters).
ALK-positive large B-cell lymphoma patient-derived xenografts. (A-E) ALK-positive large B-cell lymphoma morphology shows sheets of large neoplastic lymphoid cells with plasmablastic morphology (A), including moderate to abundant cytoplasm and round to irregular nuclei with prominent nucleoli. The neoplastic cells are positive ALK1 (B) and IgA (C), and negative for CD20 (D) and CD30 (E). (F-J) A PDX model of the ALK-positive large B-cell tumor was generated by implanting a tumor fragment underneath the kidney capsule, which generated a tumor mass after 6 to 8 weeks. The morphology of the PDX model (F) was identical to that of the patient's tumor, with strong ALK1 expression (G) and negative for CD20 (H). (I) PDX tumor replacing the implanted left kidney of mouse (right) and uninvolved right kidney on left (millimeters). (J) PDX bivalved showing tan, soft-cut surface of tumor (centimeters).
We created RCI and SCI PDX models of this tumor in NSG mice (Figure 1F-J). Engraftment was detected 4 to 6 weeks after implantation and restricted to the site of implantation in all models. Tumors comprised sheets of large neoplastic cells with morphology and immunophenotype of the original tumor, including strong expression of ALK, absence of other B- and T-cell markers (Figure 1F-H). We also confirmed the PDX and original tumor shared an identical HLA haplotype and clonotype, carried a CLTC-ALK fusion accompanied by high expression of ALK (RNA-sequencing), and exhibited very strong concordance of copy number alterations, somatic mutations and associated variant allele frequencies (supplemental Material).
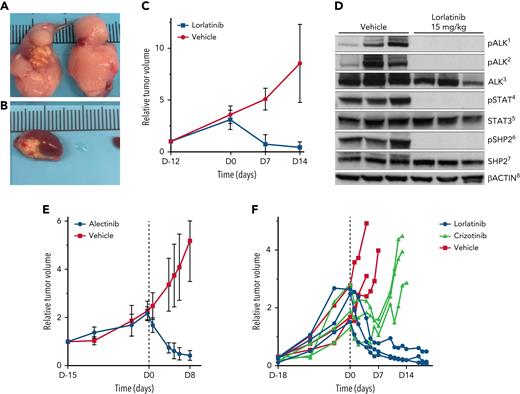
We evaluated lorlatinib in tumor volume-matched cohorts of RCI PDX mice. Lorlatinib resulted in complete abrogation of ALK phosphorylation by western blot (Figure 2D). In a cohort of mice with subrenal capsule engraftment, lorlatinib resulted in markedly reduced mean tumor volume (54.0 mm3 [range, 5.45-172 mm3] to 9.1 mm3 [range, 2.4-16.4 mm3]) after 14 days of lorlatinib. In contrast, vehicle-treated PDX tumors increased (55.1 mm3 [range, 8.6-130.6 mm3] to 398.3 mm3 [range, 77.1-888.23 mm3]; P = .0015) (Figure 2A-C; supplemental Material). We also evaluated lorlatinib and alectinib in tumor-matched cohorts of SCI PDX mice from 2 separate patients and found similar tumor volume reductions in lorlatinib- and alectinib-treated mice, and tumor volume increases in vehicle-treated mice (Figure 2E-F). After a transient initial response, tumor volume increased in crizotinib-treated mice (Figure 2F).
Lorlatinib, alectinib, and crizotinib activity in ALK-positive large B-cell lymphoma patient derived xenografts. (A-B) PDX tumors after 4 days of treatment with vehicle show tumor growth in the engrafted left kidney as well as extension and growth into the right kidney (A). (B) PDX tumors treated with lorlatinib show marked regression of tumor in the engrafted kidney in mice after 4 days of treatment with lorlatinib. (C) Lorlatinib induced rapid tumor regression in vivo in RCI PDX tumors. (D) Lorlatinib induced a complete block of ALK phosphorylation, in turn disrupting activation of downstream signaling pathways, including abrogation of STAT3 and SHP2 phosphorylation. (E) Alectinib induced rapid tumor regression in vivo in SCI PDX tumors. (F) Lorlatinib induced rapid tumor regression and crizotinib induced only transient tumor regression followed within 7 days by rapid tumor growth in vivo in SCI PDX tumors. 1, pALKTyr1604; 2, pALKTyr1278; 3, total ALK and ALK fusion proteins; 4, pSTAT3Tyr705; 5, total Stat3 protein; 6, pSHP2Tyr542; 7, total SHP2 protein; and 8, levels of total β-actin.
Lorlatinib, alectinib, and crizotinib activity in ALK-positive large B-cell lymphoma patient derived xenografts. (A-B) PDX tumors after 4 days of treatment with vehicle show tumor growth in the engrafted left kidney as well as extension and growth into the right kidney (A). (B) PDX tumors treated with lorlatinib show marked regression of tumor in the engrafted kidney in mice after 4 days of treatment with lorlatinib. (C) Lorlatinib induced rapid tumor regression in vivo in RCI PDX tumors. (D) Lorlatinib induced a complete block of ALK phosphorylation, in turn disrupting activation of downstream signaling pathways, including abrogation of STAT3 and SHP2 phosphorylation. (E) Alectinib induced rapid tumor regression in vivo in SCI PDX tumors. (F) Lorlatinib induced rapid tumor regression and crizotinib induced only transient tumor regression followed within 7 days by rapid tumor growth in vivo in SCI PDX tumors. 1, pALKTyr1604; 2, pALKTyr1278; 3, total ALK and ALK fusion proteins; 4, pSTAT3Tyr705; 5, total Stat3 protein; 6, pSHP2Tyr542; 7, total SHP2 protein; and 8, levels of total β-actin.
Encouraged by these data, we treated 4 consecutive patients with refractory, centrally confirmed ALK-LBCL (supplemental Material; p11) with next-generation ALK inhibitors. None had achieved durable complete response (CR) with prior therapies in the relapsed/refractory setting, and 3/3 were refractory to prior crizotinib. We selected alectinib based on high-potency against ALK and drug access (at the time, lorlatinib was restricted to patients with ALK-positive non-small-cell lung cancer with progressive disease after ≥2 ALKi including crizotinib).
Four consecutive patients were treated with alectinib at 600 mg PO twice daily off-label. All 4 responded including 3 CRs and 1 partial responses (supplemental Material). Response is maintained in 2 patients on continued alectinib, including 1 who underwent myeloablative allogeneic stem cell transplant (ASCT; matched related donor [MUD]), in ongoing CR at 22.3 month; one who received consolidation with 1 cycle of daratumumab/hyaluronidase-fihj in combination with alectinib, then underwent myeloablative ASCT (MUD), in ongoing CR at 7.2 months. In both patients undergoing ASCT, we did not observe acute/chronic graft versus host disease or other transplant-related complications.
Of 2 patients who developed PD, 1 subsequently received lorlatinib 100 mg PO daily off-label.4 The patient’s lymphoma symptoms resolved within days and positron emission tomography-computed tomography-confirmed CR (supplemental Material).
Of 3 patients with prior crizotinib refractoriness, post-crizotinib/pre-alectinib tissue was available for targeted DNA sequencing in 2, ALK fluorescence in situ hybridization in 1, and ALK and KRAS copy number analysis in 1 (supplemental Material). We found no ALK or KRAS amplification or ALKi resistant mutations.
In summary, ALK-LBCL has a dismal prognosis, particularly in the relapsed/refractory setting, where no long-term survivors have been reported in the literature. We encourage clinical trial participation for all patients with ALK-LBCL given no established standard of care, but clinical trials have generally been unfeasible because of the rarity of the disease, and most patients are treated with off-label therapies. Our data support use of alectinib or lorlatinib as off-label therapeutic options in relapsed/refractory ALK-LBCL.
We found that increasing ALKi potency at least partially overcomes ALKi resistance in ALK-LBCL. Additionally, lorlatinib induced CR in 1 patient who progressed on alectinib, indicating that transitioning between high-potency ALK inhibitors might also be beneficial. We did not find ALK resistance mutations or gene amplifications to be associated with crizotinib-resistant tumors. We hypothesize that crizotinib resistance in ALK-LBCL might occur via upregulation of bypass signaling pathways possibly engaged by the tumor microenvironment, and that this can be overcome with higher potency ALK inhibitors. Further studies are necessary to determine whether bypass mechanisms similar to those reported in ALKi-resistant anaplastic large cell lymphoma18 may mediate ALKi resistance in ALK-LBCL. Because crizotinib is a P-glycoprotein substrate whereas alectinib/lorlatinib are not, further studies are also necessary to determine if drug efflux via P-glycoprotein contributes to crizotinib resistance in ALK-LBCL.
Few patients achieve durable remissions/cures following frontline chemotherapy (eg, cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisolone; rituximab, etoposide, prednisolone, oncovin, cyclophosphamide; hydroxydaunorubicin). Our data warrant multicenter efforts to determine if adding alectinib/lorlatinib to first-line chemotherapy improves outcomes, and to investigate novel combinations to overcome ALKi resistance (eg, with a novel ALK degrader) through dual inhibition of ALK and key mediators of downstream signaling implicated in ALKi resistance in lymphoma (eg, PI3K-AKT,19 SHP2,18 RAS-MAPK,20 JAK-STAT effector pathways).21 Numerous PDX models of diffuse large B-cell lymphoma (DLBCL) have been reported. However, ALK-LBCL shares few biologic features of DLBCL and, to our knowledge, none of the DLBCL PDX models capture the unique biology driving ALK-LBCL. The newly generated PDX models will be key to testing novel therapies in ALK-LBCL to overcome ALKi resistance.
These data support use of off-label alectinib or lorlatinib in patients with relapsed/refractory ALK-LBCL, and allogeneic stem cell transplant should be considered for patients who achieve an adequate response. In addition, we have illustrated the potential of PDX models to inform therapeutic options, particularly for patients with rare malignancies.
Acknowledgments
The authors thank the patients and their families.
This work was made possible through the MGH Lymphoma Translational Research and Biobanking Collaborative supported by the Scott Nathan and Laura DeBonis Fund for Clinical Research (J.S.A.), and further supported by philanthropic funding from an anonymous donor (A.L.), the Jonathan Kraft Translational Research Award (J.D.S. and A.L.), and the National Institutes of Health National Cancer Institute (5R01CA196703) (R.C.).
Authorship
Contribution: J.D.S. and A.L. had full access to the data and were responsible for data collection, data analysis, and data interpretation, wrote the first draft of the manuscript, and were responsible for manuscript submission; A.R., A.D.Z., Y.W., R.K.G., A.R.E.-J., S.H., J.D., C. Mecca, A.D., H.L., C. Menard, L.Y., L.R., K.B., H.M., M.L., S.M., A.K., O.K., E.P., and R.C. were responsible for data collection, data analysis, and data interpretation, and reviewed and approved the manuscript; and M.L., V.N., and J.S.A. were responsible for data analysis, data interpretation, and reviewed and approved the manuscript.
Conflict-of-interest disclosure: J.D.S. reports consulting fees from Abbvie, AstraZeneca, Beigene, Biogen, Bristol Myers Squibb, Roche, TG Therapeutics, and Verastem, and research funding from Adaptive Biotechnologies, Beigene, BostonGene, Genentech/Roche, GlaxoSmithKline, MEI, Moderna, and TG Therapeutics. A.D. reports consulting fees from Physician’s Education Resource, Seattle Genetics, Takeda, EUSA Pharma, and Abbvie, and research funding from Roche and Takeda. M.L. reports consulting fees for AbbVie, AstraZeneca, Epizyme, Fresenius Kabi, and Intervention Insights. V.N. reports consulting fees from Lilly. J.S.A. reports consulting fees from Abbvie, Bayer, Celgene, Gilead, Juno Therapeutics, Kite Pharma, Genentech, Amgen, Novartis, Karyopharm, Verastem, Janssen, Merck, and Seattle Genetics. A.D.Z. reports consulting fees from Adaptive Biotechnologies, Abbvie, Amgen, AstraZeneca, Beigene, Bristol Myers Squibb, Celgene, Genentech/Roche, Gilead, MEI Pharma, MorphoSys, NCCN, Novartis, and Verastem, data safety monitoring committee membership for BMS, Celgene, and Juno, data safety monitoring committee membership chair for Beigene, and research funding from MEI Pharma, Gilead, Beigene, and Roche. A.K., O.K., and E.P. report employment at BostonGene. The remaining authors declare no competing financial interests.
Correspondence: Jacob D. Soumerai, Massachusetts General Hospital Cancer Center, 55 Fruit St, Boston, MA 02114; e-mail: jsoumerai@mgh.harvard.edu; and Abner Louissaint Jr, 149 13th St, Charlestown, MA 02114; e-mail: alouissaint@partners.org.
References
Author notes
∗A.R. and S.H. contributed equally to this study.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.