Abstract
Introduction AL amyloidosis is characterised by fibrillar deposition of amyloidogenic light chains causing progressive organ failure. Measurement of serum free light chains (sFLC) transformed the diagnosis and management of amyloidosis; but limitations include the inability to detect the monoclonal FLC component in some patients and difficulties in clonal assessment at low serum levels or in renal impairment. MALDI-TOF MS to measure sFLC shows promise to overcome many of these limitations. We report the first large series of prospectively followed patients with AL amyloidosis assessed using MALDI-TOF MS to detect sFLC and report the concordance with routine methods and the impact on survival outcomes and organ responses.
Methods Serum samples from patients in a prospective observational study of newly diagnosed AL amyloidosis (ALCHEMY) were analysed at diagnosis and at 6 post-diagnosis. All patients underwent serial protocolised amyloidosis assessments. Diluted serum samples were incubated with polyclonal antisera specific for κ and λ FLC conjugated to magnetic microparticles. Captured FLC were eluted and reduced, spotted onto target plates and analysed by MALDI-TOF MS. The presence or absence of monoclonal FLC was denoted as FLC-MS positive and FLC-MS negative. Landmark analysis was undertaken at 6 months.
Results 487 patients are included. The median age was 67 years (range 36 - 88) and 378 (77.9%) had ECOG performance status <2. 290 (59%) had cardiac involvement and 256 (52.4%) had renal involvement. Cardiac disease stage (Mayo European modification) was stage I - 99 (20.3%), II - 178 (36.5%), IIIa - 167 (34.2%) and IIIb - 39 (8%). 256 (52.4%) patients had ≥2 organs involved. The involved free light chain (iFLC) was κ in 90 (18.5%), λ in 395 (81.1%) and unidentified in 2 (0.41%) by Freelite assay. Median iFLC at presentation was 197mg/l (range 11.6 - 15,900mg/l) and difference between involved and uninvolved light chain (dFLC) was 177.7mg/l (range 0 - 15,898mg/l).
Based on tissue fibril typing by immunohistochemistry or proteomics 53 (10.9%) had κ AL-type and 292 (60.0%) had λ AL-type. There was 100% concordance in the light chain isotype identified by FLC-MS. Comparing FLC-MS vs. sFLC measurement by Freelite assay, 88 (97.8%) patients with a κ iFLC had a concordant κ clone by FLC-MS and 393 (99.5%) patients with a λ iFLC had a concordant λ clone by FLC-MS. 4 (0.8%) patients did not have a FLC clone detected by FLC-MS. Of patients presenting with a normal sFLC ratio, FLC-MS identified a clone in 113/114 (99.1%) patients. Using FLC-MS 26 patients (5.3%) were biclonal (κ and λ).
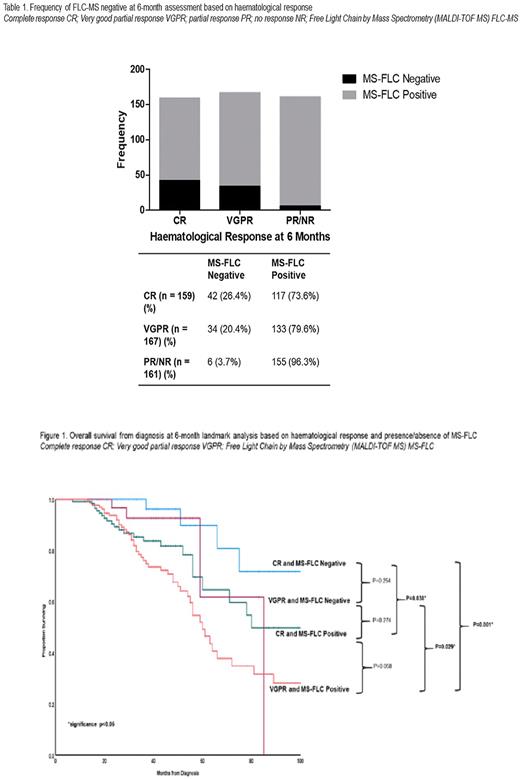
After treatment at 6 months, the haematological responses by standard ISA criteria were: complete response (CR) 159 (32.6%), very good partial response (VGPR) 167 (34.3%), partial (PR)/no response (NR) 161 (33.1%). 82 (16.8%) patients were MS-FLC negative of which 42 (26.4%), 34 (20.4%) and 6 (3.7%) patients were in CR, VGPR and PR/NR respectively (Table 1). 50/199 (25.1%) of patients with dFLC <10mg/l and 45/154 (29.2%) patients with iFLC<20mg/l were FLC-MS negative.
At 6 months, there was significantly better overall survival (OS) for patients who were FLC-MS negative vs. positive (median not reached vs. 63 months (p<0.001)). For those achieving a CR/ VGPR, there was significantly better OS for patients who were FLC-MS negative vs. positive, median OS not reached vs. 80 months (p = 0.038) and median OS 85 vs. 60 months (p = 0.029), for patients in CR and VGPR respectively (FIg.1)
261 (90%) patients with cardiac involvement and 302 (86.6%) with renal involvement were assessable for response at 6 months. In the FLC-MS (negative vs. positive patients), cardiac response was seen in 15/42 (35.7%) vs. 56/248 (22.6%) (p=0.0867) and renal response in 15/63 (23.8%) vs. 52/286 (18.2%) (p=0.453).
Conclusion Detection of clonal FLC by MS shows 100% concordance with amyloid fibril typing from tissue biopsies and can identify a FLC clone in patients presenting with a normal FLC ratio and in the majority of patients in conventional CR post treatment. After treatment, patients with no detectable residual monoclonal FLC by FLC-MS had significantly better OS compared to patients with a conventional CR/VGPR but remaining FLC-MS positive. Detailed organ response data will be presented. FLC assessment by MALDI-TOF MS represents a crucial advance in the diagnostic armamentarium for the identification and monitoring of monoclonal FLC in patients with AL amyloidosis.
Disclosures
Giles:The Binding Site: Research Funding. Berlanga:The Binding Site: Current Employment. Harding:The Binding Site: Current Employment, Membership on an entity's Board of Directors or advisory committees. Whelan:Akcea: Honoraria; Alnylam: Honoraria; Novartis: Honoraria; Pfizer: Honoraria. Gillmore:Alnylam: Consultancy; Ionis: Consultancy; Eidos: Consultancy; Intellia: Consultancy. Pratt:Binding Site: Consultancy; BMS/Celgene: Consultancy; Gilead: Consultancy; Janssen: Consultancy; Takeda: Consultancy; Amgen: Consultancy. Wechalekar:Janssen: Honoraria, Other: Travel Support; Alexion: Honoraria, Other: Travel Support; Attralus: Honoraria; Takeda: Other: Travel Support; GSK: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.