Abstract
Introduction: Pegylated interferon alfa-2a [PEG-IFN] have been used for almost 3 decades in patients with polycythemia vera [PV] and essential thrombocythemia [ET], but long-term data from prospective studies are limited.
Objective: We present results of our single center, prospective, phase II study of PEG-IFN in patients with ET and PV with a median follow up of 183 months (range, 6-198, at data lock June 2022; previous data lock was May 2015).
Methods: The study enrolled patients with ET (n=40) or PV (n=43) over the age of 18, irrespectively of previous therapy (37% untreated). PEG-IFN dose modifications, monitoring, and response assessment were as published (Masarova et al, Lancet Haematology, 2017). Testing sensitivity for JAK2V167F mutation allele burden was 1% till 2018, and 0.1% since then. Molecular response [MR; assessed in patients with baseline JAK2 ≥20%] was defined as complete (CMR; undetectable), partial (PMR; decrease by ≥50% but detectable) and minor (mMR; decrease by 20-49%).
Results: Among 83 enrolled patients (median age 53 years, 29 males), after median follow up of 183 months (range, 6-198), 11 patients (13%) continue on active therapy on study. Current doses range from 45 mcg every 9 weeks to 180 mcg weekly. Forty-six (55%), 18 (22%) and 6 (7%) patients were treated for ≥5, ≥10 and ≥15 years, respectively. The overall median exposure to PEG-IFN (n = 83) was 95 months (range, 3-191).
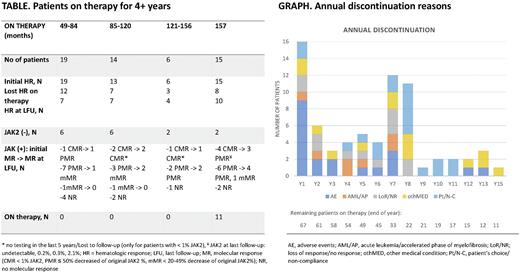
Twenty-seven patients (41%) from the initial 66 hematologic responders [HR; 80%] were in HR at the time of their last follow-up, including 5 patients who lost but regained HR with longer therapy. Table 1 shows responses in patients who remained on study for 4+ years. Median duration of HR was 137 months (range, 2.2-189). Thirty-five (63%) of 55 JAK2 mutated patients with molecular sequencing achieved molecular response: 10 CMR (18%), 20 PMR (36%) and 5 mMR (9%). At the time of last follow-up on study, 24 patients remained in MR (67%). Only 17 treated patients underwent repeated molecular sequencing since the last study update; 3 of them experienced change: 1 lost PMR (JAK2: 38%->61%), 1 lost CMR (JAK2: 0%-6%) and 1 regained PMR (JAK2: 13%->7%). Median duration on study in months [range] was 127 [25-189], 91 [11-192], 15 [2-183] and 81.5 [6-187] for JAK2(+) patients who achieved CMR (n = 10), PMR & mMR (n = 25), without MR (n = 25) and in JAK2(-) (n = 23), respectively. Five patients with previous CMR had subsequent testing, 4 of them had detectable JAK2 allele in the range of 0.1%-0.5% using more sensitive assay. Median duration of MR was unreached in patients who achieved < 1% JAK2 and it was 96 months (range, 6-155) in patients with 20-99% JAK2 allele decrease.
Reasons for therapy discontinuation included treatment-related toxicities in 20 patients (24%; 11/13 for grade ≥3), patient's decision in 19 (23%), ET/PV progression or lack of response in 12 (14%), other reasons in 10 (12%, e.g., motor vehicle accident), transformation to myelofibrosis or acute leukemia [MF/AML] in 7 (8%) and death in 4 patients (5%, all unrelated). Medical reasons for discontinuation after 10 years on study included 3 carcinomas: 1 head squamous cell, 1 pancreatic and 1 ovarian; and 2 adverse events: grade 3 Sjogren syndrome and grade 2 recurrent transaminitis. Annual discontinuation rates are shown in graph.
There were no transformations to MF/AML on therapy since the last study update for a total number of cases of 7. No additional vascular thrombotic events occurred with overall incidence of 0.9 / 100 person-years. Five patients experienced new adverse events after 10 years on therapy: mild flu-like symptoms, fatigue or diarrhea (3); grade 2 transaminitis (1) and Sjogren syndrome (1).
Twenty-five patients continued on active follow-up after PEG-IFN discontinuation until the current data lock with a median follow-up off therapy of 92 months (range, 8-132). Twenty patients are alive (on cytoreductive therapy in 11), 5 died (acute leukemia in 2, other medical condition, bleeding, stroke for 1 each). As of last follow-up, 25% (n = 21) of originally enrolled patients died. Median overall survival (n = 83) since enrollment was unreached.
Conclusions: Our 15 years of follow-up of pegylated interferon in patients with ET and PV confirms durability of responses and disease control in patients who are able to tolerate long therapy and an acceptable safety profile.
Disclosures
Bose:Sierra Oncology (now GSK): Consultancy; Blueprint Medicines Corporation: Honoraria, Research Funding; Telios: Research Funding; Astellas: Research Funding; Disc Medicine: Research Funding; NS Pharma: Research Funding; Promedior: Research Funding; Pharma Essentia: Honoraria; Novartis: Honoraria; AbbVie: Consultancy; Karyopharm: Consultancy; BMS: Consultancy, Research Funding; Cogent: Honoraria, Research Funding; Kartos: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Honoraria, Research Funding; Pfizer: Research Funding; Ionis: Research Funding; CTI BioPharma: Honoraria, Research Funding; Incyte: Honoraria, Research Funding. Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. Borthakur:Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy. Kantarjian:KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; NOVA Research: Honoraria; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; ImmunoGen: Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; Takeda: Honoraria. Verstovsek:Novartis: Consultancy, Research Funding; Constellation Pharmaceuticals: Consultancy; Roche: Research Funding; NS Pharma: Research Funding; Gilead: Research Funding; PharmaEssentia: Research Funding; Promedior: Research Funding; Protagonist Therapeutics: Research Funding; ItalPharma: Research Funding; Incyte: Consultancy, Research Funding; Sierra Oncology: Consultancy, Research Funding; Genentech: Research Funding; CTI BioPharma Corp.: Research Funding; Celgene: Consultancy, Research Funding; Blueprints Medicines Corp.: Research Funding; AstraZeneca: Research Funding; Pragmatist: Consultancy.
OffLabel Disclosure:
Pegylated interferon alfa 2a is an off-label product used in patients with polycythemia vera and essential thrombocythemia.
Author notes
Asterisk with author names denotes non-ASH members.