Abstract
Myelodysplastic syndromes/myelodysplastic neoplasms (MDS) are associated with variable clinical presentations and outcomes. The initial response criteria developed by the International Working Group (IWG) in 2000 have been used in clinical practice, clinical trials, regulatory reviews, and drug labels. Although the IWG criteria were revised in 2006 and 2018 (the latter focusing on lower-risk disease), limitations persist in their application to higher-risk MDS (HR-MDS) and their ability to fully capture the clinical benefits of novel investigational drugs or serve as valid surrogates for longer-term clinical end points (eg, overall survival). Further, issues related to the ambiguity and practicality of some criteria lead to variability in interpretation and interobserver inconsistency in reporting results from the same sets of data. Thus, we convened an international panel of 36 MDS experts and used an established modified Delphi process to develop consensus recommendations for updated response criteria that would be more reflective of patient-centered and clinically relevant outcomes in HR-MDS. Among others, the IWG 2023 criteria include changes in the hemoglobin threshold for complete remission (CR), the introduction of CR with limited count recovery and CR with partial hematologic recovery as provisional response criteria, the elimination of marrow CR, and specific recommendations for the standardization of time-to-event end points and the derivation and reporting of responses. The updated criteria should lead to a better correlation between patient-centered outcomes and clinical trial results in an era of multiple emerging new agents with novel mechanisms of action.
Introduction
Myelodysplastic syndromes (MDS; renamed recently by the World Health Organization [WHO] “myelodysplastic neoplasms”) include a biologically and clinically diverse group of hematopoietic malignancies that affect mainly older adults.1-4 Given the wide heterogeneity in clinical outcomes, a personalized, patient-centered approach to treatment and evaluation of treatment success is important.1-3 Patients are generally grouped into 2 main risk categories (lower- and higher-risk) using validated risk stratification tools, such as the International Prognostic Scoring System (IPSS), its revised version IPSS-R, and most recently, the molecular IPSS (IPSS-M).5-12
Response assessments in patients with MDS have continued to evolve over time (supplemental Table 1, available on the Blood website). The initial 2000 MDS response criteria by the International Working Group (IWG) have been used in clinical trials, regulatory reviews, and drug labels.13-15 The revised IWG 2006 criteria subsequently became the standard for response assessment.16-18 Another revision in 2018 focused on hematologic improvement (HI) for lower-risk (LR)–MDS, but the modified 2006 criteria continue to be used in trials and by regulators for the assessment of investigational agents for higher-risk (HR)–MDS.16,19 Concerns grew in the community regarding whether these criteria fully capture the clinical benefits of investigational drugs (and in some cases, potentially overestimate therapeutic benefits), whether they serve as valid surrogate measures for meaningful clinical end points, such as overall survival (OS), and their limited interrater consistency.18 Thus, we convened an international panel of 36 MDS experts and used a modified Delphi process to develop updated consensus recommendations for response assessment that are more reflective of clinically relevant outcomes in HR-MDS. Augmented by case-based examples, this paper highlights the limitations of the current IWG 2006 MDS response criteria and presents the revised IWG 2023 MDS criteria, with a focus on HR-MDS. Although the definition of HR-MDS is variable across clinical trials and routine clinical practice settings, an IPSS-R score of >3.5 is frequently used as a threshold to distinguish LR-MDS and HR-MDS based on differences in OS reported among 7212 primary untreated patients with MDS who were included in the “IWG for prognosis in the MDS” database.20 However, we acknowledge that the standard definition of HR-MDS is evolving with the wider use of molecular testing results and the incorporation of risk stratification tools such as the IPSS-M in clinical practice.10Table 1 illustrates our proposal for how to define HR-MDS in the context of the IWG 2023 MDS response criteria. On a protocol-by-protocol basis, the IPSS-M risk categories of moderate-high, high, and very high can potentially define HR-MDS pending additional prospective validation.10 We refer the reader to the earlier publication for response criteria in LR-MDS.19
Methods
An international panel of physicians participating in research and clinical care of patients with MDS was convened. This group comprised 36 experts from 14 countries across 5 continents that were invited by the core group based on their experience and contributions to the field. The core group conducted multiple virtual meetings in 2022 to develop revised criteria based on a coordinated and iterative panel review process. For each update, proposed recommendations were assessed for comment and consensus by the full panel using a modified Delphi process that involved 2 rounds of voting via an online survey following a previously established methodology.21 To develop the initial draft recommendations, a systematic literature review was performed to identify the association between the IWG 2000 and IWG 2006 response criteria with OS (supplemental Methods). Recommendation levels were classified based on the degree of agreement of the expert panel as either consensus (75% to 100% consensus) or majority agreement (50% to 74%). None of the final proposals received <75% consensus. Tables 2 and 3 provide an overview of the recommendations agreed to by the panelists and how they compare to the IWG 2006 criteria.16 Clinical case vignettes are included to highlight the limitations of the current IWG 2006 criteria and how the new IWG 2023 criteria improve upon them (Table 4).
When developing these consensus recommendations, certain guiding principles were fundamental: (1) development of data-driven recommendations to the strongest extent possible; (2) applicability to a broad, global patient and provider population with differences in resources (eg, limited availability of molecular testing results); (3) emphasis on well-validated outcomes such as complete remission (CR) while enabling the dedicated study of provisional outcomes, such as near-CR end points and molecular end points (eg, molecular clearance and measurable residual disease [MRD]); (4) practicality and inter- and intraobserver consistency of application of criteria; and (5) applicability to evolving classification and prognostication systems, especially with increasing recognition of a continuous myeloid malignancy spectrum that is driven by biology rather than arbitrary blast thresholds; consequently, having criteria that could enable reconciliation and harmonization of MDS and acute myeloid leukemia (AML) response criteria wherever possible.
Proposed IWG 2023 response criteria for HR-MDS
Response definition
Our approach to response assessment per the proposed IWG 2023 criteria is outlined in Figures 1 and 2 and supplemental Table 3, as well as illustrative cases provided in Table 4.
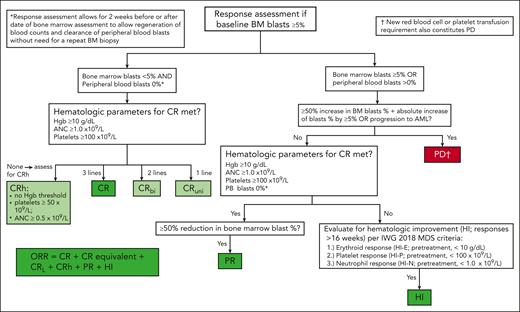
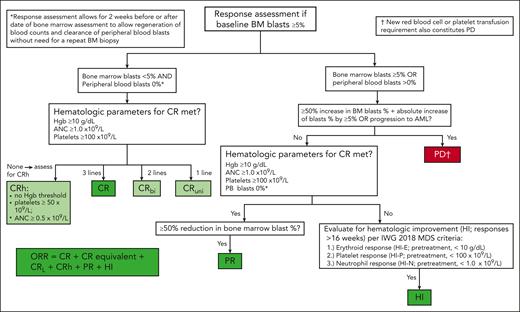
Response assessment flowchart for patients with ≥5% BM blasts at baseline. A flowchart for response assessment per the IWG 2023 response criteria is depicted. Responses shown in green (CR, CR equivalent, CRuni, CRbi, CRh, PR, and HI) are considered an objective response, whereas PD (shown in red) is considered treatment failure. CRL is a composite of CRuni and CRbi, depending on the number of lineages with cell counts at or above the threshold for CR. Of note, patients require ≥5% blasts before treatment initiation to be considered evaluable for CR, PR, CRh, or CRL but response is independent of baseline PB counts. Among patients with <5% blasts at baseline, patients who achieve hematologic recovery consistent with thresholds for CR (ie, Hb ≥ 10 g/dL, platelets ≥ 100 × 109/L, and ANC ≥ 1.0 × 109/L) as well as complete clearance of all baseline cytogenetic abnormalities should be reported as a CR equivalent and included in the ORR (see Figure 2 for details). For patients with MDS-IB2 and/or AML/MDS overlap, reporting of CRh can be considered to enhance consistency with AML trials. Both CRL and CRh are considered provisional response criteria requiring additional prospective validation (shown in light green).
Response assessment flowchart for patients with ≥5% BM blasts at baseline. A flowchart for response assessment per the IWG 2023 response criteria is depicted. Responses shown in green (CR, CR equivalent, CRuni, CRbi, CRh, PR, and HI) are considered an objective response, whereas PD (shown in red) is considered treatment failure. CRL is a composite of CRuni and CRbi, depending on the number of lineages with cell counts at or above the threshold for CR. Of note, patients require ≥5% blasts before treatment initiation to be considered evaluable for CR, PR, CRh, or CRL but response is independent of baseline PB counts. Among patients with <5% blasts at baseline, patients who achieve hematologic recovery consistent with thresholds for CR (ie, Hb ≥ 10 g/dL, platelets ≥ 100 × 109/L, and ANC ≥ 1.0 × 109/L) as well as complete clearance of all baseline cytogenetic abnormalities should be reported as a CR equivalent and included in the ORR (see Figure 2 for details). For patients with MDS-IB2 and/or AML/MDS overlap, reporting of CRh can be considered to enhance consistency with AML trials. Both CRL and CRh are considered provisional response criteria requiring additional prospective validation (shown in light green).
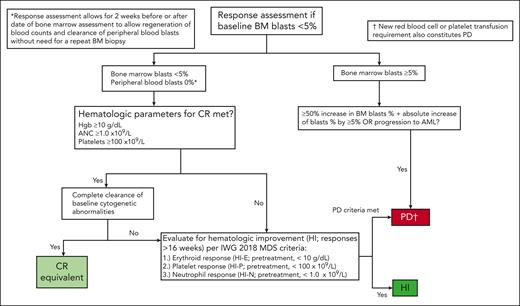
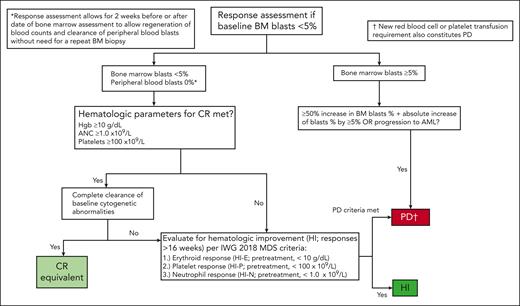
Response assessment flowchart for patients with <5% BM blasts at baseline (ie, prior to the current line of therapy). A response assessment flowchart for patients with HR-MDS with <5% BM blasts at baseline is depicted. High-risk disease status in these patients can result from high-risk cytogenetic abnormalities (eg, complex karyotype) and/or the degree of cytopenia. If a patient achieves hematologic recovery consistent with thresholds for CR (ie, Hb ≥ 10 g/dL, platelets ≥100 × 109/L, and ANC ≥1.0 × 109/L) as well as complete clearance of all baseline cytogenetic abnormalities, this should be reported as a CR equivalent. Patients who do not achieve complete cytogenetic remission (or who are not evaluable for cytogenetic clearance because of a normal karyotype at baseline) should be evaluated for HI and PD and reported as such. For patients with <5% BM blasts at baseline the definition of PD might be applied to patients with a ≥50% relative increase in BM blast count who do not have an absolute increase of ≥5% blasts in the right clinical context (eg, worsening disease-related cytopenias). Criteria for CR, HI, SD, and PD are provided in Table 1.
Response assessment flowchart for patients with <5% BM blasts at baseline (ie, prior to the current line of therapy). A response assessment flowchart for patients with HR-MDS with <5% BM blasts at baseline is depicted. High-risk disease status in these patients can result from high-risk cytogenetic abnormalities (eg, complex karyotype) and/or the degree of cytopenia. If a patient achieves hematologic recovery consistent with thresholds for CR (ie, Hb ≥ 10 g/dL, platelets ≥100 × 109/L, and ANC ≥1.0 × 109/L) as well as complete clearance of all baseline cytogenetic abnormalities, this should be reported as a CR equivalent. Patients who do not achieve complete cytogenetic remission (or who are not evaluable for cytogenetic clearance because of a normal karyotype at baseline) should be evaluated for HI and PD and reported as such. For patients with <5% BM blasts at baseline the definition of PD might be applied to patients with a ≥50% relative increase in BM blast count who do not have an absolute increase of ≥5% blasts in the right clinical context (eg, worsening disease-related cytopenias). Criteria for CR, HI, SD, and PD are provided in Table 1.
CR
The IWG 2006 criteria define CR as a reduction in bone marrow (BM) blast percentage to ≤5% and improvement in peripheral blood (PB) counts with hemoglobin (Hb) ≥11 g/dL, platelets ≥100 × 109/L, and an absolute neutrophil count (ANC) ≥1.0 × 109/L independent of baseline values.16 Although these PB count requirements are arbitrary and not linked to improvements in patient-centered outcomes, analyses of pooled data from multiple trials and our systematic review have associated achieving CR as the best response to hypomethylating agent (HMA) therapy with prolonged OS compared with “less-than-CR” responders (supplemental Figure 2A).22
The European LeukemiaNet (ELN) 2017 and 2022 response criteria for AML use a BM blast count <5% as the threshold for CR and do not include a Hb cutoff.23,24 As patients with HR-MDS are increasingly being treated on AML clinical trials and patients with oligoblastic AML (defined as 20% to 30% BM blasts) on MDS trials,25 the panel highlights the importance of trying to harmonize response criteria while emphasizing the need for an individualized approach to patients with MDS. The potential implications of discrepant response criteria were recently demonstrated by Peterlin et al, who reported the results of a trial of CPX-351 for the treatment of MDS.26 Applying ELN 2017 AML and IWG 2006 MDS response criteria led to substantial differences in response rates, which were primarily driven by the influence of Hb on achieving a CR.26 With growing evidence that some patients with MDS with blasts >10% have a prognosis similar to oligoblastic AML, leading to the proposal of an MDS/AML overlap disease category in the International Consensus Classification, this is expected to become an increasingly relevant issue.27,28
To define CR in MDS, the panel proposed the adoption of a BM blast threshold of <5% (rather than ≤5%), which is in line with IPSS-R and the 5th WHO MDS classification, which use <5% for risk stratification and the definition of MDS with low blasts, respectively.4,6 This also harmonizes the IWG 2023 MDS response criteria with the recently updated ELN 2022 AML response criteria in this respect.23
As highlighted in the IWG 2018 response criteria for LR-MDS, a Hb cutoff of ≥11 g/dL maintained over 8 weeks as a prerequisite for CR has not been demonstrated to be associated with improved survival.19 The ELN 2017 and 2022 definitions of CR in AML do not specify any Hb threshold.23,24 As the IPSS-R identified Hb <10 g/dL as an adverse prognostic factor, we propose to lower the Hb threshold for CR to ≥10 g/dL, which is highly likely to be associated with red blood cell (RBC) transfusion independence (TI) in previously transfusion-dependent patients.6 There was a debate in the panel regarding different Hb thresholds (including not requiring any Hb threshold) to define CR. However, a Hb threshold of ≥10 g/dL was chosen to reflect clinically meaningful erythroid recovery that is unlikely to be associated with ongoing RBC transfusion requirements, is attainable for patients with MDS receiving continuous myelosuppressive therapy, and also recognizes the poor prognosis of transfusion-dependent MDS.7,29-31
Recommendation
The panel proposes to change the cutoff for CR to BM blasts <5% and the Hb cutoff to ≥10 g/dL; the latter threshold is highly likely to be associated with RBC-TI in previously RBC transfusion-dependent patients. The thresholds for platelets ≥100 × 109/L and ANC ≥1.0 × 109/L remain unchanged. Of note, the aforementioned PB thresholds for CR apply independent of baseline cell counts. Only patients with ≥5% BM blasts before treatment initiation are eligible for CR assessment. However, among patients with HR-MDS with <5% blasts because of adverse cytogenetics and/or severe cytopenias, a complete cytogenetic response and full trilineage hematologic recovery (Hb ≥10 g/dL, platelets ≥100 × 109/L, and ANC ≥1.0 × 109/L) can be considered a CR equivalent but should be reported separately. Figure 2 provides a workflow for response assessment in patients with HR-MDS and <5% BM blasts at baseline. As molecular clearance has not been validated prospectively, it was not used to define CR. To qualify for a CR, patients must not have received platelet or RBC transfusions, erythropoiesis-stimulating agents, thrombopoietin mimetics, or granulocyte colony-stimulating factors in the preceding 2 weeks.
“Less-than-CR” responses
Although CR has been linked to improved survival, it may lead to an underestimation of the clinical benefit of a treatment if used in isolation.22,32,33 Treatment-associated improvements in neutrophil (>0.5 × 109/L) and platelet count (>50 × 109/L) are associated with a reduced risk of infectious and hemorrhagic complications, respectively.34-36 In contrast, the clinical benefit of a platelet count increase from, for example, 80 × 109/L to 120 × 109/L and an increase in ANC from 0.8 × 109/L to 1.3 × 109/L is unlikely to confer a substantial clinical improvement to the patient, but would still be classified as a CR per IWG 2006 criteria.16 Although CR has been shown to be associated with prolonged OS, this has not been the case for patients who achieve a reduction in BM blast count to ≤5% without HI.32 In the IWG 2006 criteria, a reduction in BM blasts to ≤5% associated with a proportional blast decrease of ≥50% from baseline is classified as a marrow CR (mCR), without requirement for any recovery in PB counts.16
Despite the limited clinical utility of mCR without HI, several contemporary clinical trials in MDS continue to use a composite of CR and mCR to report therapeutic efficacy.37,38 As such, there is concern that this can lead to an inflation of response rates that do not translate into OS benefits. For example, the phase 3 trial comparing rigosertib to best supportive care in patients with HR-MDS after HMA failure showed an overall response rate (ORR; defined as CR, mCR, partial remission [PR], and marrow PR) of 27% with rigosertib vs 17% with best supportive care but no difference in OS.39 Notably, none of the patients in either arm achieved a CR or PR.39 Inflated ORR by mCR in early-phase clinical trials may raise unattainable expectations, resulting in resource-intensive but ultimately negative phase 3 trials and delays in transformative therapies. The relevance of blast reduction in patients with MDS proceeding to allogeneic hematopoietic stem cell transplantation (allo-HSCT) continues to evolve, and whether mCR without HI before allo-HSCT is a prognostically relevant end point requires additional studies.40-42
HI has been shown to be associated with an improvement in OS in several studies.22,30,43 However, the use of HI is complicated by variability regarding the duration of HI, baseline transfusion patterns, and frequency of response assessments, resulting in the proposal of more stringent HI definitions in the revised IWG 2018 response criteria.19 Despite these limitations, both individual studies and our systematic review support an association between HI and improved OS compared with no response (supplemental Figure 2B).22,30,43,44
More recently, CR with partial hematologic recovery (CRh) as a novel response category has been accepted by the Food and Drug Administration in the regulatory approval of ivosidenib and enasidenib for relapsed/refractory IDH1 and IDH2-mutated AML, respectively.16,45-47 CRh, defined as BM blasts <5%, ANC ≥0.5 × 109/L, and platelet count ≥50 × 109/L, has also been added to the ELN 2022 response criteria for AML.23 Except for 1 retrospective study,33 CRh has not been prospectively validated in MDS, and there is currently insufficient evidence to support CRh as a new full-response criterion in HR-MDS. Further studies are warranted in patients with MDS with increased blasts or MDS/AML to determine the prognostic utility of this new response parameter in the context of MDS.
Recommendation
To recognize the importance of HI as an adjunct to morphologic BM blast response, the panel proposes to introduce CR with limited count recovery (CRL) as a provisional response category in MDS for prospective validation in place of mCR. This proposal recognizes that patients achieving HI to thresholds less than CR along with BM blast reduction have likely experienced a disease-modifying treatment effect. The specific thresholds for CRL are identical to those for CR and are similarly independent of baseline PB counts. Notably, CR has been repeatedly associated with improved OS.22,32,33,44 CRL can occur either in only 1 lineage (CR unilineage [CRuni]) or 2 lineages (CR bilineage [CRbi]) and should be reported as such (Table 2). As above, for patients with MDS/AML or MDS-IB2 as defined by the International Consensus Classification and the 5th WHO classifications, respectively, reporting CRh can be considered to achieve consistency with the ELN 2022 AML response criteria.4,23,28
“Less-than-CR” responses may also have relevance in HR-MDS if there has been a BM blast reduction along with partial but clinically relevant improvements in PB counts, such as an ANC ≥0.5 × 109/L and/or platelets ≥50 × 109/L (CRh), which are associated with lowered rates of infectious or bleeding complications.34-36 This is particularly relevant with continued dosing of MDS therapies that are myelosuppressive. Thus, the panel proposes to introduce both CRL and CRh as provisional response criteria in MDS. Similar to CR and for consistency and practicality reasons, both CRL and CRh are defined independently of the baseline PB counts. Inclusion of both CRL and CRh in the IWG 2023 criteria will allow for prospective validation and the comparison of their relative prognostic value. Figure 1 illustrates how CRL and CRh fit into the landscape of response assessment in MDS.
PR and stable disease (SD)
Per IWG 2006 response criteria, PR is defined as HI meeting the criteria for CR and a reduction of BM blasts by ≥50% compared with pretreatment to a level that remains ≥5%.14,16,48 Based on its definition, PR is primarily relevant for HR-MDS. Because of the rarity of PR responses, associations with long-term outcomes are not well characterized, but available data suggest an association with improved OS compared with nonresponders.22 The same limitations described for CR regarding the cutoffs of specific hematologic parameters also apply to PR, and it is unclear which patients with PR transition to an eventual CR or are in the early stages of relapsing disease after an incomplete response to treatment.
As the goal of therapy for most patients with HR-MDS is not curative, prolonged SD is presumably associated with better outcomes than disease progression, as it maintains quality of life and extends survival by delaying the eventual progression to AML, even if it is not a response per se. In the IWG 2006 criteria, SD is defined as neither progression nor PR or better after at least 8 weeks of treatment.16 SD is associated with improved OS compared with progressive disease (PD) or mCR in various cohort studies and conferred a prognosis similar to PR in 1 study.32,49,50 In addition, a subset of patients treated with HMA who achieve SD at 4 to 6 months may achieve a late response, including CR, with ongoing treatment, which has been associated with improved survival.51 Even if no formal response is achieved, ongoing HMA treatment may delay progression to AML and prolong OS compared with treatment discontinuation.49 The clinical significance of SD should be evaluated further. Similarly, SD may serve as a bridge to allo-HSCT in a selected subgroup of patients.52 However, dedicated studies evaluating the outcomes of patients with responses other than CR at the time of allo-HSCT are needed to better define any benefit of blast reduction without achieving a formal CR before allo-HSCT.
Recommendation
Although data are limited, PR appears to be associated with improved survival. The panel proposes to continue to report PR as a response category in clinical trials. Similar to the revised CR criteria, PR should be defined as BM blast reduction by ≥50% to ≥5% with Hb ≥10 g/dL, platelets ≥100 × 109/L, and ANC ≥1.0 × 109/L. If SD is reported, it should not be included as a component of the ORR. Among patients treated with HMA, SD may not necessarily equate to treatment failure and prompt discontinuation of therapy.
ORR
Definitions of ORR across clinical trials in MDS vary, limiting crosstrial comparisons. As outlined above, some components of the ORR (ie, mCR) are not satisfactory surrogates for OS.32 To ensure the correlation of ORR with long-term outcomes (eg, OS and event-free survival [EFS]) and to improve crosstrial comparisons, the panel proposes to uniformly define ORR in MDS as a composite of CR, CR equivalent, PR, CRL, CRh, and HI. Except for CRL, all response categories have been shown to be correlated with improved OS, suggesting that a composite ORR of these individual components would be expected to correlate with OS as well. Of note, if patients meet the criteria for multiple response categories (eg, CRL and CRh), they should only be included in the most stringent response category achieved (ie, CR > CRL > CRh > HI). A minority of the panelists felt that mCR could still have a value, especially in bridging patients to allo-HSCT, and should therefore still be reported. However, if mCR is reported, it should not be included in the ORR.
Recommendation
The ORR reported in clinical trials should be defined as a composite of CR (and its equivalent in patients with <5% BM blasts at baseline), PR, CRL, CRh, and HI, emphasizing the importance of adequate count improvement apart from blast clearance. The panel agreed that mCR and SD should not be included in the ORR as blast clearance without meaningful hematologic count recovery has not been linked to improved OS.
Cytogenetic response and MRD assessment
Cytogenetic responses have been included in both the IWG 2000 and 2006 criteria but were primarily extrapolated from studies in AML and chronic myeloid leukemia.13,16 Achieving a cytogenetic response has been associated with improved OS but does not necessarily correlate with HI and therefore CR rates among patients with MDS treated with azacitidine.53-57
MRD status has been increasingly recognized as a surrogate marker for long-term survival outcomes in both AML and MDS.58-62 However, it is important to note that MRD assessment by flow cytometry in MDS is limited by persistent dysplasia and normal or reactive cells that mimic residual disease and may not correlate with adverse survival.63,64 Therefore, alternative detection methods such as next-generation sequencing (NGS) might be more reliable in MDS and have been used in clinical trials.25,65 However, in the predominantly older MDS population, the presence of clonal hematopoiesis of indeterminate potential may limit the interpretability of NGS.66,67 More recently, MRD status at the time of allo-HSCT using various diagnostic techniques and the identification of certain high-risk molecular abnormalities such as TP53 mutations have been identified as independent determinants of patient outcome.42,68,69 Several recent studies have used MRD status to guide preemptive treatment for imminent relapse after allo-HSCT based on the well-established adverse prognostic relevance of MRD positivity.61,62,70 Because molecular testing results are not universally available, are currently not actionable for specific treatment selection for most patients, and are potentially variable across molecularly defined disease subgroups (eg, TP53 mutated MDS or treatment setting [eg, allo-HSCT vs HMA combination therapy]), the panel suggests to include molecular end points as a provisional response criterion requiring additional prospective validation.10,71,72 Clinical trials that use IPSS-M or other molecular disease characteristics as an inclusion criterion should report molecular end points routinely. This will allow further prospective validation of molecular end points.
Recommendation
As cytogenetic response has been shown to correlate with improved OS, we suggest continuing to report both complete and partial cytogenetic responses as previously defined by the IWG 2006 response criteria. MRD assessment by flow cytometry or molecular techniques such as NGS in MDS remains insufficiently validated and standardized for inclusion as a full-response criterion at this point but can be reported as a provisional response category in clinical trials.73 Although the panel acknowledges that molecular testing results are not universally available, we recommend the reporting of molecular end points whenever possible to enable further validation.
Addition of “not evaluable” as a new response category for clinical trials
As ORR and CR rate are frequent measures of efficacy in early-phase clinical trials, it is essential to standardize the denominator in the calculation of response rates. Reporting of clinical trial results should therefore be based on the intention-to-treat principle, and patients not evaluable for response (eg, due to early death, inadequate BM assessments, or withdrawal from study prior to response assessment) should be included in the denominator of response assessment analyses. Similar to the recently published ELN 2022 AML criteria,23 we propose the addition of “not evaluable” as a new response category in MDS (Table 2). The “not evaluable” category is especially important for patients enrolled in clinical trials and prospective studies and is less relevant to patients treated outside of clinical trials. Importantly, patients who are not eligible for certain response categories (eg, HI in the setting of preserved baseline PB counts) should not be included in the “not evaluable” category but rather subtracted from the denominator for the given response category.
Recommendation
All registered/randomly assigned patients should be included in the denominator of response assessment analyses in line with the intention-to-treat principle. This category may include patients yet to have a response assessment, suffering early death, exiting the study early, or those with technically suboptimal BM samples precluding assessment.
Definition of PD and disease relapse
PD
The IWG 2006 MDS response criteria classify PD as either an increase in BM blast percentage by ≥50%, a ≥50% decrement in ANC or platelet count, a reduction in Hb by ≥2 g/dL, or new transfusion dependence.16 However, the BM blast percentage is subject to significant interobserver variability and potential sampling variations across assessment techniques.74,75 The panel suggests that small absolute increases in blast percentage should not be classified as PD in the absence of other supporting data such as worsening cytopenias.
Recommendation
As the prognostic implications of a 50% increase in blast percentage, worsening cytopenias, or frank progression to AML are distinct, the panel proposes a more nuanced reporting of PD, including what specific criteria were met. As such, PD can be subdivided into either disease progression based on rising BM of PB blasts, worsening cytopenias/transfusion requirements, or progression to AML (Table 2). With the increasing use of more myelosuppressive regimens, transient cytopenias in the setting of myelosuppressive MDS treatment or alternative explanations (eg, infection and bleeding) are permitted and should not be reported as PD. Only the repeated (more than once and separated by at least 7 days) need for RBC or platelet transfusions within 8 weeks that are not related to an acute intercurrent illness (eg, sepsis) or treatment effect should be considered PD in the absence of progression by blast count. If the underlying cause of worsening cytopenias is unclear, a BM assessment should be performed to distinguish between a treatment-related effect and disease progression.
Disease relapse
The IWG 2006 MDS response criteria define disease relapse as any of the following: (1) a return to the pretreatment BM blast percentage, (2) a decrement of 50% from maximum remission/response levels in granulocytes or platelets, or (3) a reduction in Hb by 1.5 g/dL or transfusion dependence.16 However, no additional guidance on the classification of transient changes in the setting of an intercurrent disease process (eg, gastrointestinal tract bleed) is provided in the IWG 2006 MDS criteria.16 Similar to the discussion of PD, the prognostic relevance of small absolute changes in BM blasts and PB counts as well as the influence of treatment effects is unclear. To provide additional clarification, the panel proposes a more detailed classification of disease relapse with more specific criteria to avoid the misclassification and overinterpretation of small changes in PB counts and BM blasts (Table 2).
Recommendation
Disease relapse based on BM blasts should be defined as an absolute increase in BM blasts by ≥5% and a ≥50% increase from prior assessment, or confirmed (ie, persistent for 4 weeks and not explained by a nondisease-related process, such as infection, growth factor use, or BM recovery) reappearance of blasts in the blood, or decrement in PB counts defined as any of the following: ≥50% decline from maximum remission/response levels in granulocytes or platelets, a reduction in Hb by 1.5 g/dL or transfusion dependence, or development of extramedullary disease (myeloid sarcoma).
Definition of time-to-event–based outcomes
Although OS remains the most important outcome in clinical trials in MDS, it is influenced by multiple factors, including subsequent therapies, and often requires an extended period of follow-up to accrue the required number of events. Therefore, EFS, leukemia-free survival, and progression-free survival (PFS) have been used as secondary end points in clinical trials; however, the definitions used are heterogenous, limiting comparability across trials.18 As such, we propose standardized definitions for clinical trials (Table 3). The emphasis for all these definitions is that the time to an event should be reported for all patients enrolled on a clinical trial (intention-to-treat analysis) and be measured from the time of trial enrollment (or randomization) until the event of interest is reached. For patients treated outside of a clinical trial, time-to-event end points should be reported from the time of treatment initiation.
Although 6-month and median PFS have been proposed as surrogates for OS in MDS, PFS end points require additional validation.18 However, with expanding treatment options and increased sequencing of multiple lines of therapy, PFS and EFS warrant further study and should be included in clinical trials as secondary end points and validated as surrogate outcomes for OS.
Recommendations
OS should remain the primary end point for phase 3 clinical trials in MDS. EFS and PFS can potentially serve as surrogate outcomes for OS but require additional prospective validation. In general, time-to-event–based outcomes should be defined for all patients in a trial and measured from the date of study entry (or randomization) to the date of the event.
Definition of PRO end points
A patient-reported outcome (PRO) can be defined as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.”76 Therefore, PROs may include multidimensional concepts such as health-related quality of life or more specific concepts such as fatigue or other symptoms. The selection of the most appropriate PRO measure (or a combination of measures) for a clinical trial depends on various aspects, and the rationale for selecting a specific measure should be reported in the study protocol.77 The US Food and Drug Administration78 has recently recommended the assessment of the following core PROs in cancer clinical trials: (1) disease-related symptoms, (2) symptomatic adverse events, (3) overall side effect impact summary measure, (4) physical function, and (5) role function. International recommendations for including PROs in trial protocols and for transparent reporting in study publications are also available.79,80 Similarly, health-related quality of life end points have recently been defined as one of the core outcomes by leading European and Israeli MDS experts.81 We emphasize the importance of high-quality PRO data collection and reporting in study results.82 Many methodological issues should be carefully considered when assessing and analyzing PROs in clinical trials. For example, ignoring missing PRO data during the analyses may lead to biased conclusions about the changing of PROs over time and also about the between-treatment differences.77
Recommendation
The panel recommends the inclusion of PROs as end points in phase 2 and 3 trials.
Practical considerations
We also provide specific practical recommendations related to several aspects of the proposed IWG 2023 response criteria for HR-MDS (Table 5), such as timing of response assessment and enumeration of blasts in blood and BM, to ensure reliable reporting of outcomes across trials, and enhance interobserver consistency of reporting results. As previously suggested for LR-MDS, although a 16-week screening period for transfusion needs is preferable,19 the panel recognizes that, given the acuity of HR-MDS, an 8-week screening period before treatment initiation and a 16-week time window for the assessment of HI and TI duration are acceptable. Response assessment per the IWG 2023 response criteria for HR-MDS allows a window of 2 weeks either before or after the date of BM assessment to allow for regeneration of blood counts without the need for a repeat BM biopsy to confirm the response. The specific thresholds for neutrophil count, platelet count, and Hb level do not have to be all met on the same date but must be met within the 2-week window of the BM assessment. The date of the achieved response would be the date of the BM assessment. To qualify for a CR, CR equivalent, PR, CRh, or CRL, the patient must not have received supportive intervention for the specific lineage(s) of the response (eg, platelet and/or RBC transfusions, erythropoiesis-stimulating agents, thrombopoietin mimetics, or granulocyte colony-stimulating factors) in the preceding 2 weeks. For example, for CRuni in the platelet lineage, the patient must not have received platelet transfusions for the previous 2 weeks of achievement of the required platelet threshold (≥100 × 109/L) but could have potentially received RBC transfusions or growth factor support.
Appropriate timing of response assessment is especially important in the setting of ongoing myelosuppressive therapy. Similarly, there might be a discrepancy between <5% blasts in the BM and PB blasts being >0% at the time of BM assessment. In this setting, a repeat PB blast assessment within 2 weeks should be done to distinguish whether this elevation of PB blasts is disease related or not (eg, secondary to marrow recovery, infection, etc). If PB blasts clear (ie, are 0%) within 2 weeks of the BM biopsy/aspirate and BM biopsy/aspirate previously showed <5% blasts, the patient will have achieved a CR (in case of hematologic recovery) without the need for a repeat BM assessment for confirmation purposes.
Conclusions
The IWG 2006 response criteria in MDS have been an important tool to advance clinical research by harmonizing response assessment. However, these criteria in their current form have significant limitations, especially with regards to the definition of hematologic recovery, which does not necessarily correlate with patient-centered outcomes and OS. In the IWG 2023 criteria, we propose significant modifications to the IWG 2006 response criteria to better capture clinically relevant outcomes, reduce discrepancies with AML response criteria, and improve applicability to novel therapies. We hope that these updated criteria will lead to a better correlation between patient-centered outcomes and clinical trial results in an era of multiple emerging new agents. Future research should focus on the standardization and validation of MRD assessment, molecular and less-than-CR responses, other surrogate end points that predict OS, and the evaluation of response criteria across treatment settings (eg, after frontline therapy vs allo-HSCT).
Acknowledgments
The authors acknowledge Alyssa Grimshaw (Harvey Cushing/John Hay Whitney Medical Library, Yale University) for her support with the systematic review as well as Pierre Peterlin for his contributions to the manuscript.
This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy (Investigator Grant #20125; AIRC 5x1000 project #21267) (L.M.) and (Investigator Grant # IG-26537) (V.S.); Cancer Research UK (L.M.); the Fundación Científica de la Asociación Española Contra el Cáncer (FC AECC) and AIRC under the International Accelerator Award Program (project #C355/A26819 and #22796) (L.M.). A.M.Z. is a Leukemia and Lymphoma Society Scholar in Clinical Research.
Authorship
Contribution: A.M.Z., M.S., and J.P.B. wrote the initial draft of the manuscript. A.M.Z., U.P., J.P.B., M.S., M.A.S., and P.F. constitute the steering committee of the International Working Group 2023 Myelodysplastic Syndrome response criteria and were involved with the conception and design of the study; and all authors were involved in consensus voting, writing, reviewing, and editing the manuscript and approved the final version for submission.
Conflict-of-interest disclosure: A.M.Z. received research funding (institutional) from Celgene/Bristol Myers Squibb (BMS), AbbVie, Astex, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, and ADC Therapeutics; participated in advisory boards and/or had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene/BMS, Jazz, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Cardiff Oncology, Takeda, Ionis, Amgen, Janssen, Epizyme, Syndax, Gilead, Kura, Chiesi, ALX Oncology, BioCryst, Notable, Orum, and Tyme; and served on clinical trial committees for Novartis, AbbVie, Gilead, BioCryst, ALX Oncology, Geron, and Celgene/BMS. U.P. received honoraria from BMS, Jazz Pharmaceuticals, AbbVie, and Geron. M.S. consulted for Curis Oncology and Boston Consulting; served on the advisory board for Novartis, Kymera, Sierra Oncology, and GSK; and participated in GME activity for Novartis, Curis Oncology, Haymarket Media, and Clinical Care Options. L.A. received honoraria from BMS, Takeda, Jazz Pharmaceuticals, AbbVie, and Novartis and received research funding (institutional) from Celgene/BMS and AbbVie. R.B. received research funding and honoraria from AbbVie, BMS, and Taiho and research funding from Takeda. F.E. received consulting fees from AbbVie, Incyte, Janssen, and Syros outside the submitted work. G.S. participated in advisory boards and/or had a consultancy with and received honoraria from AbbVie, Celgene/BMS, Janssen-Cilag, Novartis, Roche, and Takeda and received grants/research supports (institutional) from Celgene/BMS, Novartis, and Gamida Cell. H.E.C. has been on speaker and advisory board activities and received honoraria from BMS, Celgene, Novartis, Jazz, and Stemline and has research support from Celgene for an investigator-initiated clinical trial. A.E.D. received consulting fees from Geron, BMS, Novartis, Takeda, Geron, Taiho, CTI Biopharma, and Gilead. O.O. has served on the advisory board of BMS, Celgene, Novartis, Taiho, and Kymera Therapeutics; serves on a data and safety monitoring board for Threadwell Therapeutics; and received research funding (institutional) from AbbVie, Agios, Aprea, Astex, AstraZeneca, BMS, Celgene, CTI, Daiichi, Incyte, Janssen, Kartos, Novartis, NS-Pharma, and Oncotherapy Sciences. A.B. received consulting or advisory board honoraria from Novartis, Acceleron, Agios, AbbVie, Takeda, Celgene/BMS, Keros Therapeutics, Taiho, and Gilead; has research support from the National Institutes of Health SPORE in Myeloid Malignancies and from the Edward P. Evans Foundation. R.K. served on the speaker bureau of Jazz Pharmaceuticals, Servio, CTI, PharmaEssentia and served on advisory boards and received honoraria from BMS, Novartis, AbbVie, Jazz Pharmaceuticals, Servio, PharmaEssentia, Taiho, Geron, and CTI. A.G.K. reports consultancy fees/honoraria/speaker bureau fees from Achillion, Akari, Alexion, AstraZeneca Rare Disease, Amgen, Apellis, Biocryst, Celgene, F. Hoffmann-La Roche, Novartis, Pfizer, NovoNordisk, Samsung, and Ra Pharma and research funding from Celgene/BMS and Novartis. R.H. has a consultancy with Bluebird Bio. U.G. received speaker honoraria from Celgene and Novartis; institutional research support from Celgene, Novartis, AbbVie, and Jazz Pharmaceuticals; and consulting fees from Celgene. J.S.G. has served on advisory boards for AbbVie, Astellas, and Genentech and receives institutional research funding from AbbVie, AstraZeneca, Genentech, Pfizer, and Prelude. A.H.W. has served on advisory boards for Novartis, AstraZeneca, Astellas, Janssen, Amgen, Roche, Pfizer, AbbVie, Servier, Gilead, BMS, Shoreline, Macrogenics, and Agios; receives research funding to the Institution from Novartis, AbbVie, Servier, Janssen, BMS, Syndax, Astex, AstraZeneca, and Amgen; serves on speaker bureaus for AbbVie, Novartis, BMS, Servier, and Astellas; is an employee of the Walter and Eliza Hall Institute (WEHI); and is eligible for financial benefits associated with payments which WEHI receives in relation to venetoclax. N.D. has received research funding from Daiichi Sankyo, BMS, Pfizer, Gilead, Sevier, Genentech, Astellas, Daiichi Sankyo, AbbVie, Hanmi, Trovagene, FATE Therapeutics, Amgen, Novimmune, Glycomimetics, Trillium, and ImmunoGen and has served in a consulting or advisory role for Daiichi Sankyo, BMS, Arog, Pfizer, Novartis, Jazz, Celgene, AbbVie, Astellas, Genentech, ImmunoGen, Servier, Syndax, Trillium, Gilead, Amgen, Shattuck Labs, and Agios. E.A.G. received honoraria from AbbVie, Alexion Pharmaceuticals, Genentech, Novartis, CTI Biopharma, Apellis, Celgene/BMS, Takeda Oncology, Taiho Oncology, Physician Educational Resource, MediCom Worldwide, American Society of Hematology, Picnic Health, and AAMDSIF and research support (institutional) from Astex Pharmaceuticals, Genentech, Blueprint Medicine, Alexion Pharmaceuticals, Apellis, BMS/Celgene, and Celldex Therapeutics. A.A.v.d.L. reports research grants from Celgene/BMS, Roche, Alexion and fees speaker and advisory board activities from Novartis, Amgen, Takeda, Syros, and Celgene/BMS. V.S. served on advisory boards for AbbVie, BMS/Celgene, Geron, Gilead, Menarini, Novartis, Takeda, Servier, and Syros. P.S. received grant research support from Pfizer and Alnylam; is a consultant for AbbVie, Roche Alexion, Janssen, Pfizer, BioCryst, and AstraZeneca; and speaker for Novartis, Pfizer, Alexion, AstraZeneca, Amgen, and BMS. M.A.S. has served on advisory boards for BMS, Novartis, Kurome, and Gilead. P.F. received research funding from BMS, AbbVie, Jazz Pharmaceuticals, Novartis, and Janssen and had a consultancy with and received honoraria from BMS, AbbVie, Jazz Pharmaceuticals, and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Amer M. Zeidan, Hematology Section, Department of Internal Medicine, Yale School of Medicine, Yale University, 333 Cedar St, PO Box 208028, New Haven, CT 06520-8028; e-mail: amer.zeidan@yale.edu.
References
Author notes
∗A.M.Z. and U.P. are joint first authors and contributed equally to this study.
†M.A.S. and P.F. are joint senior authors and contributed equally to this study.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.