Abstract
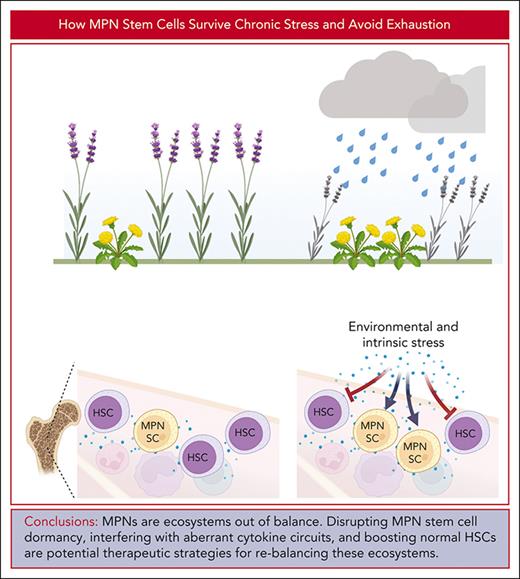
Chronic or recurrent episodes of acute inflammation cause attrition of normal hematopoietic stem cells (HSCs) that can lead to hematopoietic failure but they drive progression in myeloid malignancies and their precursor clonal hematopoiesis. Mechanistic parallels exist between hematopoiesis in chronic inflammation and the continuously increased proliferation of myeloid malignancies, particularly myeloproliferative neoplasms (MPNs). The ability to enter dormancy, a state of deep quiescence characterized by low oxidative phosphorylation, low glycolysis, reduced protein synthesis, and increased autophagy is central to the preservation of long-term HSCs and likely MPN SCs. The metabolic features of dormancy resemble those of diapause, a state of arrested embryonic development triggered by adverse environmental conditions. To outcompete their normal counterparts in the inflammatory MPN environment, MPN SCs co-opt mechanisms used by HSCs to avoid exhaustion, including signal attenuation by negative regulators, insulation from activating cytokine signals, anti-inflammatory signaling, and epigenetic reprogramming. We propose that new therapeutic strategies may be derived from conceptualizing myeloid malignancies as an ecosystem out of balance, in which residual normal and malignant hematopoietic cells interact in multiple ways, only few of which have been characterized in detail. Disrupting MPN SC insulation to overcome dormancy, interfering with aberrant cytokine circuits that favor MPN cells, and directly boosting residual normal HSCs are potential strategies to tip the balance in favor of normal hematopoiesis. Although eradicating the malignant cell clones remains the goal of therapy, rebalancing the ecosystem may be a more attainable objective in the short term.
Introduction
Human hematopoiesis generates ∼108 leukocytes, 2 × 108 erythrocytes, and 1011 platelets daily to support oxygen transport, hemostasis, and immune response.1 Demand for mature hematopoietic cells can increase suddenly because of injury or infection, necessitating a system with large spare capacity and high regenerative potential. To address this challenge, complex regulatory networks maintain balance between quiescence, proliferation, self-renewal, and differentiation. Mouse data suggest that chronic inflammation or recurrent episodes of acute inflammation can exhaust the hematopoietic system’s regenerative capacity prematurely and compromise its function.2 In humans, chronic inflammation can aggravate cytopenias in inherited bone marrow failure (BMF) syndromes, suggesting that inflammation may contribute to BMF.3 The opposite seems to apply in myeloid malignancies and their precursor clonal hematopoiesis (CH; the presence of bona fide driver mutations in the blood or BM of people without obvious hematologic abnormalities4). Starting with our initial observations, a large body of data has accumulated to implicate inflammation as a critical progression driver in myeloid malignancies, particularly myeloproliferative neoplasms (MPNs), raising the question of how malignant clones avoid attrition in highly inflammatory environments.5 Here, we review the mechanisms that protect normal hematopoiesis from exhaustion under inflammatory stress, drawing parallels between chronic inflammation and continuously increased proliferation in MPNs. This is followed by an in-depth discussion of dormancy as a crucial mechanism to preserve hematopoietic stem cells (HSCs) under stress, considering the possible role of stochastic effects. We then cover how myeloid malignancies co-opt physiological mechanisms to avoid extinction, illustrating general principles with selected examples. Lastly, we review emerging clinical implications and potential therapeutic strategies.
Stress effects on hematopoiesis
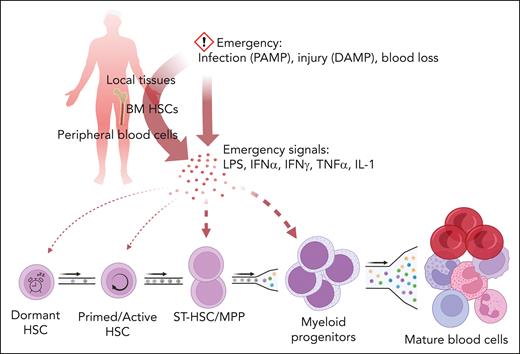
At homeostasis, blood cell numbers and lineage composition are maintained within narrow boundaries, with individual variation reflecting genetic and environmental factors.6 Increased demand for mature blood cells triggers a coordinated response termed emergency hematopoiesis that involves a complex, context-dependent interplay between pathogen-associated molecular pattern molecules (PAMPs), cytokines, and hematopoietic and stromal cells (reviewed in7). For instance, short-term HSCs (ST-HSCs) and multipotent progenitor cells (MPPs) express high levels of toll-like receptors (TLRs) and can be directly stimulated by PAMPs such as lipopolysaccharide (LPS), the ligand for TLR4, whereas nonhematopoietic TLR4-expressing cells are required for LPS-induced, granulocyte colony-stimulating factor (G-CSF)-mediated myelopoietic responses.8,9 Barcoded HSC transplants in mice and data from human gene therapy trials suggested that steady state hematopoiesis is maintained by small numbers of long-term HSCs (LT-HSCs) that continuously undergo self-renewal divisions, producing a steady stream of maturing blood cells.10,11 However, barcoding of individual HSCs using Cre recombinase or sleeping beauty transposase subsequently showed that multiple clones contribute to homeostatic hematopoiesis under nontransplant conditions, and identified ST-HSCs and MPPs as the main sources of mature blood cells, suggesting that, in homeostasis, the ST-HSC compartment requires minimal contributions from upstream LT-HSCs to remain balanced.12,13 In contrast, flux from the LT-HSC to downstream compartments is much greater after treatment with 5-fluorouracil, suggesting that LT-HSCs are recruited to maintain downstream compartments under stress (Figure 1).13
Activation of emergency hematopoiesis. Emergency hematopoiesis is initiated by PAMP, damage-associated molecular patterns (DAMP), or acute blood loss that trigger release of cytokines from hematopoietic stem and progenitor cells (HSPCs), mature blood cells, and nonhematopoietic bystander cells. Emergency cytokines activate hematopoiesis in a manner commensurate to the strength of the signal, with the main burden of proliferation/differentiation borne by ST-HSCs and MPPs, whereas dormant HSCs are shielded.
Activation of emergency hematopoiesis. Emergency hematopoiesis is initiated by PAMP, damage-associated molecular patterns (DAMP), or acute blood loss that trigger release of cytokines from hematopoietic stem and progenitor cells (HSPCs), mature blood cells, and nonhematopoietic bystander cells. Emergency cytokines activate hematopoiesis in a manner commensurate to the strength of the signal, with the main burden of proliferation/differentiation borne by ST-HSCs and MPPs, whereas dormant HSCs are shielded.
Chronic inflammatory stress in normal hematopoiesis
Hematopoiesis handles single acute stress events without significant reduction of LT-HSCs, whereas chronic or repeated acute inflammation can lead to HSC attrition.2,14-17 In fact, chronic infections are in the differential diagnosis for patients with pancytopenia, and a wealth of data from animal studies support the notion that chronic inflammation can have profound effects on HSC function. LT-HSCs from mice challenged by chronic infection (Mycobacterium avium-intracellulare [MAI]) or repeated exposure to PAMPs (such as poly I:C) exhibit reduced repopulation capacity that resembles aged hematopoiesis, suggesting sustained inflammatory stress reaches the most primitive cells.2,18 Various cytokines and PAMPs are implicated in mediating inflammatory effects on HSCs, including interleukin-1β (IL-1β), IL-6, and LPS, among others.17,19 Moreover, there is a widespread role for type I or type II interferon (IFN) signaling, depending on the specific inflammatory stimuli and their corresponding pattern recognition receptors. In MAI infection models, LT-HSC dormancy exit is abrogated by genetic ablation of type II IFN signaling (Ifngr1−/−or Stat1−/−), but not type I IFN signaling (Ifnar−/−). Although LT-HSC numbers in Ifng−/− and Ifng+/+ are similar, Ifng−/− HSCs exhibit improved engraftment, suggesting an increase in dormant cells.18 Subsequent studies confirmed that IFN-γ signaling causes HSC exhaustion in chronic bacterial and viral infections,20,21 whereas in poly I:C challenge models, type I IFN signaling promotes recruitment of dormant LT-HSCs.22,23 In addition, chronic viral infections can profoundly affect the BM stroma. For example, CXCL12 abundant reticular cells are decimated in mice with chronic lymphocytic choriomeningitis as the result of IFN-α and IFN-γ production by virus-specific CD8+ T cells.24 Aging and experimentally enforced HSC proliferation induce hypermethylation of polycomb repressive complex (PRC)-regulated hematopoietic lineage genes.2,25 Altogether, although evolution has devised effective mechanisms to protect the core of hematopoiesis during organismal lifetime, defenses can be breached by persistent stress. As such, CH and MPNs could be regarded as adaptive responses to inflammatory stress, a notion discussed in subsequent sections.26,27 Because studies in mice suggest that flux from the dormant LT-HSC to downstream compartments is minimal, one would predict that damage to dormant HSCs becomes apparent only when the compensation capacity of downstream compartments is exhausted (Figure 1).12 This would explain why most cases of aplastic anemia lack an exposure history, but, to our knowledge, has not been tested experimentally. With the limitations of normal HSCs to handle repeated acute or chronic inflammatory stress, how do SCs in myeloid malignancies manage to avoid exhaustion? To approach this question, we will compare inflammatory signaling in MPNs and physiological stress responses.
Chronic proliferation stress in MPN hematopoiesis
MPNs and chronic infections share clinical features, including constitutional symptoms, left-shifted granulopoiesis, and elevated inflammation biomarkers. These clinical commonalities are paralleled by similarities in the pathways activated by intrinsic (oncogenic) or extrinsic (inflammatory) signals. However, compared with HSCs, MPN SCs are more resilient or may even exhibit paradoxical responses to cytokines, generating a survival differential between cell populations living in the same ecosystem.
Mimicry of inflammatory cytokine signaling
Many MPNs are driven by mutations that activate pathways downstream of cytokine receptors such as JAK-STAT, MAPK, and nuclear factor κB.28-30,CSF3R (also known as G-CSF receptor) mutations illustrate the complex relationships between physiological and oncogenic signaling. Short term G-CSF induces HSC proliferation; however, this is limited to CD41+ cells with transient regenerative potential, whereas dormant HSCs are mobilized but not induced to proliferate.31 Accordingly, short term G-CSF–induced transcriptional changes, although indicative of cell cycle activation, are mild.32 In contrast, long-term G-CSF exposure reduces HSC repopulation.33 Chronic G-CSF treatment of patients with severe congenital neutropenia (SCN) is associated with acquisition of truncating CSF3R mutations that activate Src kinase signaling and predate transformation to myelodysplastic syndrome/acute myeloid leukemia (AML), a frequent complication of SCN.34,35 Recent work from the Touw laboratory showed that these mutations confer G-CSF hypersensitivity to normal, but not to SCN-derived, induced pluripotent stem cells, explaining why long-tern G-CSF does not lead to HSC exhaustion.36 In contrast, membrane-proximal CSF3R mutations characteristic of chronic neutrophilic leukemia (CNL) activate JAK/STAT, similarly to physiological G-CSF signaling.35 Thus, to avoid exhaustion, CNL leukemic stem cells (LSCs) may depend on additional mutations that activate self-renewal, explaining why CNL with isolated CSF3R mutations is rare.37
Inflammatory milieu
MPN and normal HSCs inhabit an ecosystem containing their own progeny, cells of the innate and acquired immune system, nonhematopoietic cells, and noncellular components.38-40 Elevated inflammatory cytokines, are characteristic of MPNs,41-43 associated with various mutations, and contributed by bidirectional interactions between MPN and normal cells.5,44-46 Differential sensitivity to specific cytokines is implicated in CH progression to clonal dominance. For instance, tumor necrosis factor α (TNF-α) signaling through TNF receptor 1 (TNF-R1) promotes expansion of mutant HSCs in Dnmt3aR882H/+ transgenic mice.47 TNF-α signaling through TNF-R2 promotes expansion of JAK2V617F mutant human and mouse HSPCs.5,48 To which degree normal HSCs survive in inflammatory MPN ecosystems differs between subtypes. In chronic myeloid leukemia (CML), BCR::ABL1 inhibitors can induce cytogenetic responses, even in long-standing disease, and chemotherapy is followed by mobilization of BCR::ABL1− cells, but this is not seen in other MPNs.49,50 Whether residual normal HSCs are irreversibly exhausted or can be resuscitated will define the limits of nontransplant therapies.
Infections, commensal microbiota, and immunity
Registry studies found that prior infection is associated with a higher risk of myelodysplastic syndromes/AML51 and MPN.51,52Tet2+/+ mice that received transplantation with a mix of Tet2−/− and Tet2+/+ BM develop an incompletely penetrant MPN that is limited to those animals with demonstrable 16S bacterial rRNA gene in hematopoietic organs, suggesting host and environmental factors influence clonal expansion.53 Autoimmune disorders also increase the risk for myeloid neoplasms, with the excess risk emphasizing entities without detectable antibodies, such as Crohn disease, hinting at cellular immunity as the driver.52 Given the wealth of genome wide association studies on autoimmune diseases, it may be of interest to mine this data for potential associations with myeloid malignancies.54
Dormancy
MPN SCs exhibit impaired cell cycle control. For instance, transcriptional profiling revealed that, unlike their normal counterparts, quiescent CML LSCs are set to cycle.55 The activity of TP53, a potent inducer of quiescence, is often reduced in the chronic phase of MPNs, with inactivating mutations limited to blast phase. Accordingly, chronic phase-CML LSCs are sensitive to combined disruption of p53 and Myc.56,57 As forced cell-cycle entry due to knockout of cell cycle regulators such as Cdkn2 can cause HSC exhaustion, MPN SCs rely on mechanisms to fine-tune cell cycle control (Figure 2).58 During the transition from homeostatic to emergency hematopoiesis and vice versa, the fundamental cell fate decisions between quiescence vs cell cycle entry and between self-renewal vs differentiation are recalibrated. To ensure long-term maintenance of a fully functional hematopoietic system, robust mechanisms preserve LT-HSC numbers and functionality.59 Dormant HSCs are at the core of this highly resilient system. In homeostasis, most LT-HSCs are quiescent. Pulse-labeling studies using 5-bromo-2′-deoxyuridine or histone 2B (H2B)–green fluorescent protein revealed that the quiescent LT-HSC compartment comprises a large pool of non–label-retaining cells (NLRC) and a much smaller pool of label-retaining cells (LRCs) (Figure 3A).60-62 Quiescent NLRCs are set to cycle, evidenced by upregulation of positive (eg, Cdk6) and downregulation of negative cell cycle regulators (eg, p57), whereas opposing patterns characterize LRCs. Upon cytokine stimulation, LRCs enter cell cycle with longer latency compared with NLRCs, indicating greater distance from cell cycle entry, a state referred to as dormancy. Dormant LT-LSCs undergo only 4 to 5 divisions over the life of a mouse, remember each division, and contain the entire long-term repopulation activity. Which mechanisms control this striking cell division limit is unknown.61-63 Single-cell RNA sequencing demonstrated a continuum of transition states between NLRC and LRC, allowing response in proportion to signal strength (Figure 3B).64 Numerous transcription factors are implicated in the regulation of quiescence and dormancy, of which p53, Foxo family, and Myc seem the most important (Table 1).
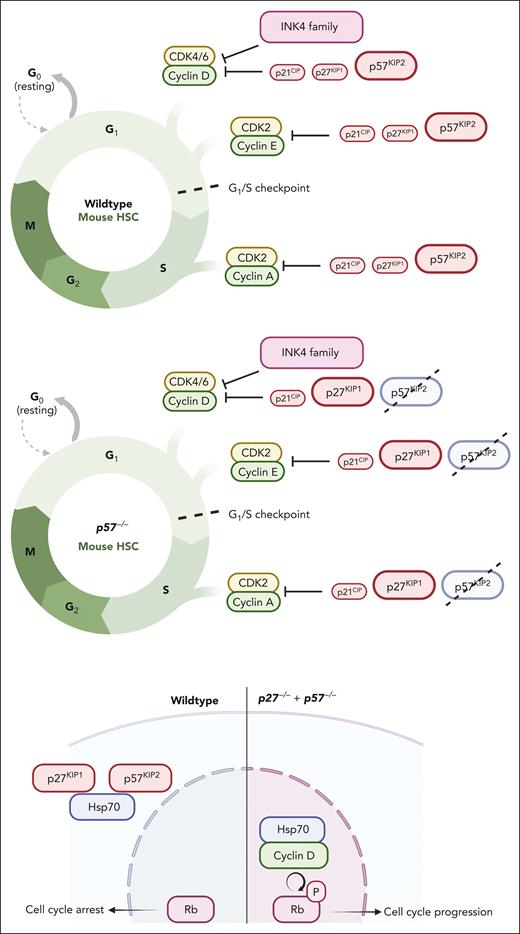
Cell cycle regulation in HSCs and MPN SCs. Progression through the G1 checkpoint into S phase is regulated by the retinoblastoma protein (Rb), whose activity is controlled via phosphorylation by cyclin dependent kinases (CDKs) that are activated by D cyclins, and negatively regulated by CDK inhibitors. Total CDK activity rather than substrate specificity orderly drives the cell cycle progression.65 In HSCs, this critical system is tightly regulated, with redundant checks and balances. For instance, cell cycle entry is normal in p57 (Cdkn1c)−/− HSCs because of compensatory upregulation of p27. However, HSCs null for both p57 and p27 show increased proliferation and reduced engraftment when transplanted. Mechanistically, Hsp70 interacts with cytoplasmic p57 and p27. Combined deletion of p57 and p27 from HSCs results in nuclear translocation of an Hsp70/cyclin D1 complex that promotes Rb phosphorylation.66 The role of specific cell cycle regulators varies between mouse strains. For instance, p21 (Cdkn2a) deletion causes hematopoietic failure in serial transplantation in 129/Sv, but not in C57BL/6 mice, unless the latter are irradiated.67,68
Cell cycle regulation in HSCs and MPN SCs. Progression through the G1 checkpoint into S phase is regulated by the retinoblastoma protein (Rb), whose activity is controlled via phosphorylation by cyclin dependent kinases (CDKs) that are activated by D cyclins, and negatively regulated by CDK inhibitors. Total CDK activity rather than substrate specificity orderly drives the cell cycle progression.65 In HSCs, this critical system is tightly regulated, with redundant checks and balances. For instance, cell cycle entry is normal in p57 (Cdkn1c)−/− HSCs because of compensatory upregulation of p27. However, HSCs null for both p57 and p27 show increased proliferation and reduced engraftment when transplanted. Mechanistically, Hsp70 interacts with cytoplasmic p57 and p27. Combined deletion of p57 and p27 from HSCs results in nuclear translocation of an Hsp70/cyclin D1 complex that promotes Rb phosphorylation.66 The role of specific cell cycle regulators varies between mouse strains. For instance, p21 (Cdkn2a) deletion causes hematopoietic failure in serial transplantation in 129/Sv, but not in C57BL/6 mice, unless the latter are irradiated.67,68
Label retention and cell cycle entry upon initiation of emergency hematopoisis. (A) After pulse-labeling with green fluorescent protein (GFP) or 5-bromo-2′-deoxyuridine (BrdU), a small fraction of HSCs retain high levels of label (LRCs), indicating a state of continued quiescence (dormancy). Almost all repopulation potential resides within the dormant population. (B) Continuous transition states exist between dormant and actively cycling HSCs, allowing the hematopoietic emergency response to be adjusted to the signal strength. The dormant HSC compartment is resilient to acute stress but can be exhausted under chronic stress.
Label retention and cell cycle entry upon initiation of emergency hematopoisis. (A) After pulse-labeling with green fluorescent protein (GFP) or 5-bromo-2′-deoxyuridine (BrdU), a small fraction of HSCs retain high levels of label (LRCs), indicating a state of continued quiescence (dormancy). Almost all repopulation potential resides within the dormant population. (B) Continuous transition states exist between dormant and actively cycling HSCs, allowing the hematopoietic emergency response to be adjusted to the signal strength. The dormant HSC compartment is resilient to acute stress but can be exhausted under chronic stress.
Metabolic characteristics of dormant HSCs
Late relapses in patients with AML or the fact that only ∼30% of patients with CML achieve treatment-free remission speak to the recalcitrance of dormant LSCs.95 Understanding dormancy regulation may be central to understanding how LSCs survive in inflammatory environments. Although both dormant and quiescent HSCs are in G0, metabolic properties distinguish dormancy from mere quiescence.
Energy metabolism
In contrast to NLRC, LRCs exhibit low oxidative phosphorylation (OXPHOS) and low glycolysis,96 challenging the view that glucose usage within the LT-HSC compartment is universal.97-99 Rather than glucose, dormant HSCs may use fatty acids, amino acids, and nucleic acids to meet energy needs.100-103 HSCs with low mitochondrial membrane potential, consistent with low OXPHOS, have the highest repopulating potential.96,104
Protein homeostasis (proteostasis)
Protein synthesis in dormant HSCs is low and tightly regulated by intrinsic and extrinsic factors; increasing or decreasing protein synthesis compromises HSC repopulation capacity.105 Several mechanisms are implicated in fine-tuning HSC proteostasis that converge on the translation machinery, with a central role for the PI3K/AKT/mTOR axis. HSCs null for the PI3K antagonist Pten exhibit increased protein synthesis but reduced long-term repopulation potential. Deletion of the mTOR activator Rictor in Pten−/− HSCs normalizes protein synthesis, and improves repopulation, suggesting Pten deletion depletes HSCs by increasing protein synthesis.106 Similarly, protein synthesis is reduced in mice with a hypomorphic mutation in the Rpl24 ribosome subunit (Rpl24Bst/+). Rpl24Bst/+ HSCs exhibit reduced engraftment that is restored by combining Rpl24Bst/+ with Pten−/−107. The transcription factor LAG1 inhibits translation initiation by 4E-BP through complex mechanisms, promoting HSC expansion.105,108 Interestingly, some extrinsic signals elicit differential responses in HSCs and hematopoietic progenitor cells (HPCs), optimizing protection of HSCs, while maximizing differentiated cell output. Angiogenin, an RNase secreted by niche cells, is endocytosed by HSPCs and promotes generation of transfer RNA–derived stress-induced small RNA (tiRNA) in HSPCs but of ribosomal RNA in differentiated progeny. Consequently, protein synthesis is reduced in HSPCs but increased in differentiating cells, promoting HSC quiescence but myeloid progenitor proliferation.109 Stressors, such as changes in redox balance or energy status, induce endoplasmic reticulum stress and the unfolded protein response.110 In response to endoplasmic reticulum stress, HSCs undergo apoptosis, whereas HPCs adapt and survive.111 Together, these data support a critical function of proteostasis for HSC maintenance, and, by proxy, for regulation of dormancy.
Autophagy and lysosomal metabolism
A third metabolic pillar of HSC dormancy is autophagy, the transport of cytoplasmic materials to lysosomes for degradation and/or reuse. Deletion of autophagy genes such Atg7 promotes mitochondrial metabolism in quiescent HSCs, leading to cell cycle entry, differentiation, and exhaustion.112-115 Cytosolic proteins that contain a KFERQ motif form complexes with HSP70, which interact with lysosomal-associated membrane protein 2 for translocation into lysosomes, a process referred to as chaperone-mediated autophagy.116 Chaperone-mediated autophagy promotes protein quality control and balanced energy metabolism, and its age-related decrease contributes to functional HSC decline.117 However, decline is not universal, as one-third of aged HSCs resemble young HSCs, with high autophagy, low metabolism, and robust regeneration potential.
Dormancy and diapause
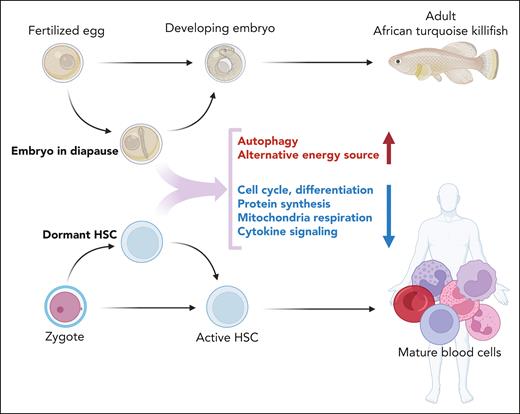
Pathways governing metabolic responses to environmental cues, such as mTOR are often evolutionarily conserved, suggesting that important insights may be derived from other species responding to metabolic stress.118 Many aspects of dormancy resemble diapause, a program of suspended embryonic development used by selected species during unfavorable environmental conditions.119,120 Diapause arrests the embryo at the blastocyst or even late embryonic stage, delaying development.121 In contrast to senescence in aging SCs, diapause is reversible. There are striking similarities between the factors regulating entry to, and exit from, dormancy and diapause, respectively. Deletion of both c-Myc and n-Myc from embryonic SCs reduces transcription, splicing, and protein synthesis, leading to a reversible growth arrest with retained pluripotency that transcriptionally resembles that of diapaused epiblasts.122 Similarities extend to PRC1 and PRC2, epigenetic master regulators implicated in hematopoietic development and LT-HSC maintenance. The PRC1 component BMI1 promotes HSC maintenance in postnatal tissues, partly by inhibiting the expression of p16Ink4a and p19Arf, tumor suppressors associated with senescence.123 Hematopoietic-specific deletion of Bmi1 slowly depletes HSCs, possibly reflecting erosion of a quiescent HSC pool. Surprisingly, deletion of p16Ink4a and p19Arf failed to correct the defect, suggesting involvement of mechanisms other than senescence.124 Indeed, Bmi1 deficiency increased protein synthesis, protein aggregation, and protein ubiquitylation independently of its effects on senescence regulators.124 Mice with deletion of Eed, a core subunit of PRC2, fail to maintain LT-HSC, and develop BMF soon after birth.125 These data parallel studies in African turquoise killifish (Figure 4), which have adapted to intermittent drying of their habitat by evolving desiccation-resistant eggs that remain in diapause in the dry mud for months but complete development within weeks once rains resume.126 Diapause in killifish is associated with profound upregulation of CBX7 and EZH1, members of PRC1 and PRC2. The similarities between dormancy and diapause are striking but questions remain: is dormancy simply a low-energy state or an active metabolic program? What triggers the return from cycling to dormancy? Of particular interest in the context of this review, some cancer cells, including AML cells, survive chemotherapy by activating diapause-like programs.127-129 In a critical experiment, mice were injected with cells from a barcoded colorectal cancer cell line. Tumors recurring after a temporary response to chemotherapy retained barcode complexity, which suggests that clonal survival is not genetically determined but either random or because of a specific functional state.128 Given the widespread dysregulation of PRC in myeloid malignancies, similarities between dormancy and diapause may point to fruitful future studies to identify strategies to eliminate dormant LSCs.
Diapause and dormancy. Some species can arrest embryonal development to circumvent adversarial environmental conditions, a program referred to as diapause. Development is resumed when conditions are favorable. Data from the turquoise African killifish suggest that diapause is an active process that maintains a specific metabolic program. Dormant HSCs are in a state of deep quiescence far removed from cell cycle entry that shares features of diapause.
Diapause and dormancy. Some species can arrest embryonal development to circumvent adversarial environmental conditions, a program referred to as diapause. Development is resumed when conditions are favorable. Data from the turquoise African killifish suggest that diapause is an active process that maintains a specific metabolic program. Dormant HSCs are in a state of deep quiescence far removed from cell cycle entry that shares features of diapause.
MPN SCs co-opt physiological mechanisms of HSC sustenance
Normal hematopoiesis can attenuate stress at multiple levels, including cytokine secretion, cytokine-receptor engagement, receptor endocytosis, signal amplification, and signal duration, allowing for a fine-tuned emergency response that protects HSCs. MPN SCs co-opt these mechanisms to avoid exhaustion, win clonal competition, and establish disease (Table 2). How can some HSCs and LSCs remain dormant in the presence of activating cytokines? Certain adhesion molecules and cytokines, such as N-cadherin, jagged-1, and angiopoietin-1 contribute to quiescence maintenance, but the most extensively studied quiescence enhancer is transforming growth factor β (TGF-β).130-132 Canonical signaling downstream of TGF-β involves activation of SMADs, but additional components contribute to a system of bewildering complexity.133 For instance, TGF-β signals to JNK through TAK1, a MAP kinase with a role in stress and inflammation signaling, and Tak1−/− mice develop BMF.134,135 Mice with hematopoietic-specific deletion of Tif1g, another noncanonical TGF-β target, develop a chronic myelomonocytic leukemia (CMML)-like MPN upon aging. This MPN, characterized by reduced LT-HSCs but increased ST-HSCs, MPPs, and granulocyte-macrophage progenitors, is transplantable, suggesting that maintained canonical TGF-β signaling is sufficient to avoid exhaustion. TIF1G is epigenetically silenced in one-third of human CMML, and its expression restored with hypomethylating agents.136 Cytokine signaling can also be attenuated at the level of receptor endocytosis. The tetraspanin MS4A3 marks myeloid lineage cells and is induced upon HSC differentiation.137 We discovered that MS4A3 promotes common β chain (βc) cytokine (IL-3/granulocyte-macrophage colony-stimulating factor) receptor signaling by escorting receptor endocytosis to enhance signaling.138 Low MS4A3 in CML LSCs reduces response to βc cytokines, preventing exhaustion.57 Similarly, lysosomal degradation of membrane receptors promotes LT-HSC quiescence, consistent with a critical role for the endo-lysosomal pathway.139 In contrast, Myc-driven repression of lysosomal catabolism activates LT-HSCs, validating Myc’s role for switching between quiescence/dormancy and proliferation.139
Downstream of stress signaling, transcriptional output orchestrated by chromatin modifiers, transcription factors, and RNA processors determines HSC fate between quiescence and cycling. Because each signaling pathway mobilizes multiple regulatory factors and vice versa, the decision between quiescence or self-renewal must reflect the sum of multiple signaling inputs.192 Somatic mutations in epigenetic modifiers such as TET2, ASXL1, IDH1/IDH2, EZH2, and DNMT3A are common in myeloid malignancies and have a profound impact on cell fate decisions and dormancy. As a comprehensive review of this subject is beyond the scope of this manuscript, we have focused on PRC1/2 to illustrate the importance of balancing effects on SC maintenance vs proliferation. Clearly, hematopoiesis has evolved multiple mechanisms to insulate dormant HSCs from extrinsic stimulation, ensuring a high threshold for cell cycle entry to protect the most valuable cells from unnecessary proliferation.
A role for stochastic processes?
Differences in methylation levels and patterns cause stochastic transcriptional heterogeneity among genetically identical cells.193,194 It is conceivable, but not experimentally proven, that this translates into differences in quiescence and repopulating potential that may insulate a portion of HSCs from stress.195-197 HSC heterogeneity likely involves additional mechanisms of expression control (eg, RNA splicing), posttranslational modifications, and signaling oscillation. Heterogeneous states may explain divergent outcomes following HSC transformation by the same oncogene. For instance, NRASG12D is a CH mutation, a founder in CMML and AML, and a single driver in juvenile myelomonocytic leukemia, validating its ability to drive clonal expansion.198,199 However, NRASG12D alone causes senescence, raising the question of how NRASG12D-expressing HSCs circumvent extinction.200 Label retention studies showed that NrasG12D expression can either increase proliferation and reduce self-renewal or the exact opposite,201 suggesting that expansion of an NRASG12D mutant clone may depend on a specific cellular state. Can LSCs transition between states that do or do not permit access to dormancy? If this were the case, it would explain why barcode heterogeneity is preserved in some cancer cells persisting during chemotherapy.127,129 Characterizing the state from which leukemic cells access dormancy may inform new therapeutic strategies.
Spontaneous remissions
Most reported spontaneous remissions of solid tumors were associated with infection and attributed to pathogen-stimulated antitumor immunity.202 The rare spontaneous remissions of established MPN involve various genotypes, including BCR::ABL1, FLT3ITD, and JAK2V617F, and likely reflect involvement of host immunity.203-207 However, the detection of BCR::ABL1 transcripts in the blood of healthy volunteers and the subsequent discovery of CH are evidence that mutant clones frequently arise.208,209 It is intriguing to speculate that many more mutant clones become exhausted before they reach detectability. Advances in simultaneous single-cell genotyping and phenotyping may make this question accessible to experimental interrogation.
Rebalancing the MPN ecosystem
Dormant MPN SCs are intrinsically resistant to therapies aimed at proliferating cells. Some effective drugs may induce quiescence, the best example being tyrosine kinase inhibitors in CML, which not only fail to eliminate, but increase, quiescent primitive CML cells.210-212 Can we use our understanding of quiescence to develop new therapies? As a starting point we propose to consider MPN hematopoiesis as an ecosystem thrown out of balance by an invasive species. Although eradicating the invaders remains the most desirable outcome, in complex myeloid neoplasms, a more realistic goal may be to rebalance the system to maintain function by weakening invasive species, strengthening resident species, or both.213 In the last section we will review several possibilities that might accomplish this.
Targeting cytokine circuits
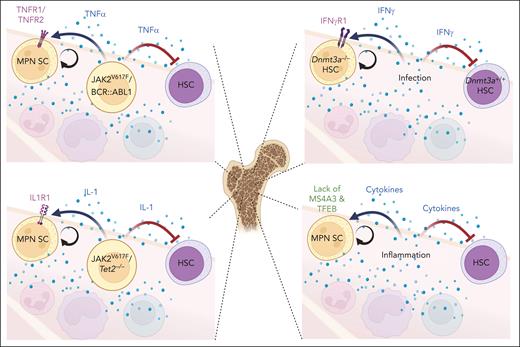
Because elevated inflammatory cytokines are an MPN hallmark, numerous cytokine-mediated interactions exist in the MPN ecosystem (Figure 5), but it is largely unknown which of these are critical for MPN progression. The most compelling evidence may exist for TNF-α. MPN cells expressing JAK2V617F or BCR::ABL1 generate TNF-α, which inhibits HSCs but is neutral to, or even stimulates, MPN SCs.5,178 Although in JAK2V617F-positive MPN, the signal is transduced through TNF-R2,48 TNF-R1 is implicated in Dnmt3aR882H/+-driven CH,47 suggesting that the effect of blocking either TNF-R1 or TNF-R2 may depend on the genetic context. Context dependency extends to the biological setting. In mice with chronic MAI infection, Dnmt3a−/− HSCs outcompete Dnmt3a+/+ cells in an IFN-γ–dependent manner, suggesting that blocking IFN-γ signaling may suppress mutant clones in the setting of chronic infection.27,47 As an emergency signal, IL-1β enhances HSC division and differentiation but chronic IL-1β exposure reversibly reduces HSCs.214 A retrospective analysis of a cardiovascular prevention trial of the IL-1β–blocking antibody canakinumab showed the greatest benefit in patients with TET2-mutated CH.215 Because IL-1β secreted by Tet2−/− monocytes promotes AML growth, canakinumab may restrain MPN cells.216,217 Clearly, at this point we are only scratching the surface of the complex cytokine networks in myeloid malignancies.
Potentially targetable cytokine circuits in MPNs. In the BM environment of MPNs and CH, inflammatory cytokines generated by mutant cells or induced by infection/inflammation influence the self-renewal and competitive fitness of both MPN SCs and HSCs (represented by arrows in figure). Differential responses of MPN SCs and HSCs to certain cytokines present potential strategies of reversing the competitive differential between MPN and normal cells to rebalance the MPN ecosystem.
Potentially targetable cytokine circuits in MPNs. In the BM environment of MPNs and CH, inflammatory cytokines generated by mutant cells or induced by infection/inflammation influence the self-renewal and competitive fitness of both MPN SCs and HSCs (represented by arrows in figure). Differential responses of MPN SCs and HSCs to certain cytokines present potential strategies of reversing the competitive differential between MPN and normal cells to rebalance the MPN ecosystem.
Disrupting MPN SC insulation and overcoming dormancy
Another angle may be to disrupt MPN SCs’ insulation from cell cycle–inducing signals to drive them out of dormancy. This is nothing new, because cytokine priming as a strategy to mobilize quiescent LSCs into cycle has been evaluated in clinical trials of AML and CML, with limited success.218,219 If studies on normal hematopoiesis in mice are predictive, these interventions may not have reached dormant LSCs that have adapted to an inflammatory, cytokine-rich environment. Exploiting the specific mechanisms used by LSCs may be more promising. For instance, small-molecule inhibitors of transcription factor TFEB may disrupt self-insulation by restoring surface expression of cytokine receptors on LSCs.139 TGF-β antagonists such as luspatercept may restore responsiveness to cytokines.139,220 We have shown that delivery of MS4A3 protein to CML CD34+ cells promotes differentiation and reduces colony formation.138 If induction and maintenance of dormancy are active processes, this will offer therapeutic opportunities. For instance, dormancy in AML cells is vitamin A dependent, and antagonizing vitamin A may drive dormant AML LSCs into cycle.64 Dormant LSCs may rely neither on glycolysis nor on OXPHOS, but may be dependent on amino acids.221 Understanding how this metabolic program is initiated could identify vulnerabilities. If dormancy in hematopoiesis is induced by master switches, in analogy to PRC1 regulation of diapause in killifish, then identifying stress sensors and pathways that transmit the dormancy command may inform strategies to eliminate dormant LSCs.
Boosting normal HSCs
IFN-α is active in polycythemia vera (PV), essential thrombocythemia, and early-stage myelofibrosis, and in the pretyrosine kinase inhibitor era, was the only nontransplant therapy known to induce cytogenetic responses in CML.222,223 IFN-α drives dormant HSCs into cycle, suggesting that cytogenetic responses may reflect mobilization of normal hematopoiesis.22,224 Indeed, this is supported by genetic mouse models, as well as studies in primary MPN cells.23,225,226 Whether clinical responses to IFN-α indeed reflect mobilization of normal HSCs or specific suppression of MPN cells remains debatable. Recent work identified a pathway involving PKCδ-dependent phosphorylation of ULK1 with subsequent activation of p38 MAPK as essential for IFN-α–suppressive effects on malignant erythroid precursors from patients with MPN. Supporting the clinical relevance of this observation, ULK1 and p38 MAPK levels positively correlate with response to IFN-α therapy in humans.227
There are more opportunities for interventions aimed at rebalancing the MPN ecosystem, such as strategies targeting interactions between MPN SCs and the BM stroma. Success may depend on the fitness of remaining normal HSCs but we know little about these cells. For instance, are they epigenetically reprogrammed after spending years in an inflammatory environment? If so, is this program reversible? Directing more research at the state of residual HSCs may be fruitful.
Perspective
Few would disagree that eradicating the malignant clone is the most desirable outcome of MPN therapy. However, the realization that CH is widespread and universal in older individuals has blurred the boundary between physiological age–associated processes and malignancy. Oligoclonality is prevalent in many aging tissues and could be seen as a mechanism that mitigates aging-related functional decline of tissue SCs. In the case of CH, the situation is further complicated by the discovery of a seemingly limitless number of medical conditions whose incidence, clinical course, or responsiveness to therapy are affected by CH, although studies with limited size must be interpreted with caution.228 More than any other organ, hematopoiesis is repeatedly subjected to extreme proliferation stress, forcing evolution to develop effective strategies to protect HSCs from damage and exhaustion. As MPNs must solve the same problem, it does not come as a surprise that MPN SCs use mechanisms used by normal HSCs to avoid exhaustion. Eliminating dormant MPN SC while sparing their normal counterparts will be challenging and may require targeting state transitions rather than the state of dormancy itself. For instance, preventing LSCs stressed by therapy from transitioning into dormancy may eliminate residual leukemia. In fact, maintaining MYC expression in AML cells treated with chemotherapy may accomplish this.127 A deeper understanding of the mechanisms triggering state transitions should reveal additional options. In the meantime, rebalancing the perturbed ecosystem of MPN hematopoiesis may be a more achievable goal, and this may include avoiding interventions that promote the outgrowth of clinically aggressive clones by suppressing targetable but nonlethal clones.229 Clinical management of myeloid malignancies is likely to become more complex.
Acknowledgments
Given the limited space, many relevant publications could not be included, and the authors apologize to those authors whose important work is not cited. Figures were generated using BioRender.
This work was supported in part by National Institutes of Health grants R01CA268496, R01CA257602, and R01CA254354 (M.D.).
Authorship
Contribution: H.G.Z. and M.D. reviewed literature, designed the structure of the review, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Deininger, Versiti Blood Research Institute, 8727 W Watertown Plank Rd, Milwaukee, WI 53226; e-mail: mdeininger@versiti.org.