Key Points
Conversion from MRD− to MRD+ or from MRD+ to MRD− status during ixazomib or placebo maintenance modulates the risk of disease progression.
Ixazomib prolonged progression-free survival in patients who were MRD+ before maintenance and at a 14-month landmark analysis.
Abstract
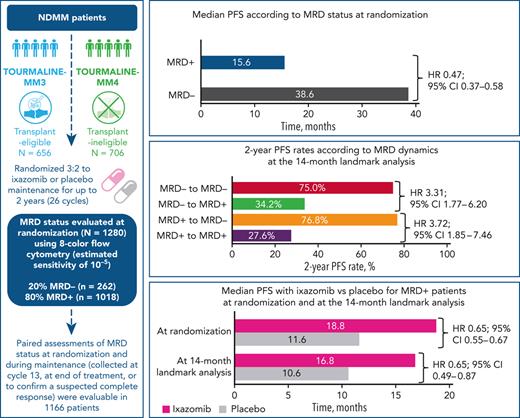
Measurable residual disease (MRD) evaluation may help to guide treatment duration in multiple myeloma (MM). Paradoxically, limited longitudinal data exist on MRD during maintenance. We investigated the prognostic value of MRD dynamics in 1280 transplant-eligible and -ineligible patients from the TOURMALINE-MM3 and -MM4 randomized placebo-controlled phase 3 studies of 2-year ixazomib maintenance. MRD status at randomization showed independent prognostic value (median progression-free survival [PFS], 38.6 vs 15.6 months in MRD− vs MRD+ patients; HR, 0.47). However, MRD dynamics during maintenance provided more detailed risk stratification. A 14-month landmark analysis showed prolonged PFS in patients converting from MRD+ to MRD− status vs those with persistent MRD+ status (76.8% vs 27.6% 2-year PFS rates). Prolonged PFS was observed in patients with sustained MRD− status vs those converting from MRD− to MRD+ status (75.0% vs 34.2% 2-year PFS rates). Similar results were observed at a 28-month landmark analysis. Ixazomib maintenance vs placebo improved PFS in patients who were MRD+ at randomization (median, 18.8 vs 11.6 months; HR, 0.65) or at the 14-month landmark (median, 16.8 vs 10.6 months; HR, 0.65); no difference was observed in patients who were MRD−. This is the largest MM population undergoing yearly MRD evaluation during maintenance reported to date. We demonstrate the limited prognostic value of a single–time point MRD evaluation, because MRD dynamics over time substantially impact PFS risk. These findings support MRD− status as a relevant end point during maintenance and confirm the increased progression risk in patients converting to MRD+ from MRD− status. These trials were registered at www.clinicaltrials.gov as #NCT02181413 and #NCT02312258.
Introduction
Measurable residual disease (MRD) status is one of the most powerful prognostic factors in newly diagnosed and relapsed multiple myeloma (MM).1,2 Persistent MRD in the setting of continuous therapy for transplant-ineligible3,4 and relapsed patients,5 as well as after induction and intensification in transplant-eligible patients,6-8 is significantly associated with inferior survival. There are promising, yet scarce, data on the clinical value of MRD assessment during continuous8-10 or fixed-duration6 maintenance therapy. In contrast, there is virtually no information on patients’ MRD status during observation.10 Paradoxically, maintenance and observation are the disease settings where MRD status is anticipated to help tailor treatment duration.11,12
Historically, MRD has been evaluated at specific single time points during therapy. However, interest is growing in the use of serial assessments to improve risk stratification based on MRD dynamics.13 Patients attaining sustained MRD negativity for 1 year or more show superior outcomes compared with those with shorter durations of MRD remission.5,6,8,9 It is therefore reasonable to hypothesize that measuring MRD kinetics at different time points is required to guide treatment intensification, cessation, or reinstatement, particularly in the maintenance and observation settings. As noted above, existing data on serial MRD assessments during maintenance are limited to a few studies in relatively small samples of patients,6,8,9 or to a 6-month interval in the large Myeloma XI trial.10 Because current treatment paradigms are based on continuous treatment until progressive disease (PD), there is very limited information on the prognostic value of MRD kinetics in patients not receiving active treatment, with only 1 reported analysis to date.10
Ixazomib has been investigated as maintenance therapy in MM, both in transplant-eligible (TOURMALINE-MM3; clinicaltrials.gov number NCT02181413)14 and transplant-ineligible (TOURMALINE-MM4; NCT02312258) patients.15 We performed a pooled analysis of these 2 global, randomized, and placebo-controlled phase 3 studies of single-agent ixazomib maintenance in patients with newly diagnosed MM.14,15 To date, this represents the largest series of patients undergoing serial MRD evaluation during maintenance therapy or placebo. Based on the trial designs, we aimed to evaluate progression-free survival (PFS) according to MRD dynamics, the volatility of MRD status over time and its clinical impact, the prognostic value of late MRD conversions from positive (MRD+) to negative (MRD−) status, the time from MRD reappearance to PD in patients receiving ixazomib vs placebo, and PFS benefit with ixazomib vs placebo according to MRD status at randomization and during maintenance.
Methods
Study designs and patients
Study designs and methodologies for the randomized, double-blind, placebo-controlled phase 3 TOURMALINE-MM3 and TOURMALINE-MM4 trials have been reported previously.14,15 In MM3, patients were enrolled in 167 study sites across 30 countries, and in MM4 patients were enrolled in 187 sites across 34 countries. The studies were similarly designed. Briefly, patients were randomized 3:2 to receive maintenance therapy with 3 mg oral ixazomib or matching placebo on days 1, 8, and 15 of 28-day cycles for up to 2 years (26 cycles). The dose was increased to 4 mg from cycle 5 if tolerated during cycles 1 to 4. Adult patients (≥18 years of age) with symptomatic MM per International Myeloma Working Group criteria who had achieved at least a partial response (PR) before maintenance randomization and had an Eastern Cooperative Oncology Group performance status of 0 to 2 were eligible.14,15 In MM3, patients who had received a proteasome inhibitor (PI) and/or immunomodulatory drug (IMiD) induction therapy followed by a single autologous stem cell transplantation (ASCT) within 12 months of diagnosis were screened ≥75 days after ASCT, and randomized ≤15 days after screening and ≤115 days after ASCT. Patients were stratified by induction regimen (PI without IMiD vs IMiD without PI vs PI and IMiD), preinduction International Staging System (ISS) disease stage (I vs II or III), and response after transplantation (complete response [CR] or very good partial response [VGPR] vs PR).14 In MM4, patients who were transplant-ineligible or unwilling/unable to undergo ASCT and had 6 to 12 months of any standard-of-care induction therapy were randomized ≤60 days after their last dose of induction. Patients were stratified by induction regimen (PI-containing vs non-PI therapy), ISS disease stage (I or II vs III) at diagnosis, age at randomization (<75 vs ≥75 years), and response before randomization (CR or VGPR vs PR).15
In both studies, the primary end point was PFS (PD or death per independent review committee [IRC] evaluation) from randomization. Prespecified secondary end points included the frequency of conversion from MRD+ to MRD− status, sustained MRD negativity, and the correlation between MRD status and survival.14,15
The trials were conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and appropriate regulatory requirements. Local ethics committees and institutional review boards approved the protocols. Every patient provided written informed consent.
MRD assessment
Bone marrow (BM) aspirates were collected for MRD evaluation from patients in CR (and/or VGPR in MM3) at randomization, cycle 13, and end of treatment (∼24 months, if no treatment delays [equivalent to 26 cycles, to the nearest complete cycle]), unless already collected within the previous 2 cycles. BM aspirates were also collected to confirm a newly suspected CR at any time point.
Samples were assessed by means of 8-color flow cytometry with antibodies against CD38-FITC (clone HB7; BD Biosciences), CD81-PE (clone JS-81; Pharmingen), CD45-PerCPCy5.5 (clone 2D1; BD Biosciences), CD138-APC (clone MI15; BD Biosciences), CD19-AF700 (clone HIB19; Pharmingen), CD27-BV510 (clone L128, BD Biosciences), CD56-V450 (clone B159; BD Biosciences), and CD117-BV605 (clone 104D2; BD Biosciences), as described previously.14
The assay was validated and standardized across 4 countries in 3 geographic regions (Americas, Europe, and Asia-Pacific) by a central laboratory (supplemental Figure 1, available on the Blood website). For the validation process, fresh EDTA-anticoagulated whole blood samples were incubated with red blood cell lysing solution, washed, and resuspended in 0.5 mL phosphate-buffered saline–bovine serum albumin (PBS-BSA). Samples were counted on a Sysmex (or equivalent) hematology instrument, and 10 million cells were transferred into a test tube. The appropriate volume of antibodies was added to the sample, which was then incubated for 20 minutes in the dark at room temperature (gentle vortex motion applied to mix the sample at the 10-minute mark). Two mL BD FACS Lysing solution was added to the test tube, vortexed and incubated in the dark for 10 minutes. Samples were centrifuged, resuspended in 1 mL PBS-BSA, and acquired within 30 minutes on a BD FACSCanto II instrument. A 5-minute water tube was acquired before each sample. The target goal for acquisition was set at a total of 5 million cells, so that an estimated sensitivity of 4 × 10−6 could be reached (ie, detectable MRD defined by a cluster of 20 clonal plasma cells among 5 × 106 nucleated cells).
Statistical methods
Kaplan-Meier methodology was used to estimate PFS distributions, with stratified log-rank tests and Cox models used for between-group comparisons. Stratification factors included study (MM3 vs MM4), preinduction ISS stage (I vs II vs III), induction regimen (PI-containing or not), and prior response (CR or VGPR vs PR). PFS, defined as time from randomization to PD or death, was analyzed according to MRD status before randomization. In addition, the effect of baseline covariates on PFS was evaluated in a separate multivariate analysis using a stratified Cox proportional hazard regression model, with study (MM3 vs MM4) as a stratification factor. PFS was also analyzed based on MRD dynamics over time. To address the immortal time bias, the analyses were landmarked at the end of 14 and 28 months. These landmarks were chosen based on the distribution of evaluable posttreatment MRD samples, in accordance with the schedule of BM aspirates described previously. The last evaluable MRD assessment from months 1 and 14 and months 15 and 28 were selected. Because of immature follow-up, analyses of the impact of MRD dynamics on overall survival were not possible.
In the absence of evaluable MRD assessment, an imputation method was used for patients’ MRD status. At randomization, patients with less than a CR (per IRC evaluation) who were missing MRD data were imputed as MRD+; patients in CR (per IRC evaluation) without MRD data were classified as “missing.” If MRD assessments were not available for the landmarks, data were imputed based on the last IRC response from months 1 to 14 and months 15 to 28, using the same imputation rule at randomization.
Results
MRD evaluation and patient characteristics
A total of 2077 BM aspirates were analyzed across the 2 studies. Overall, 101 BM aspirates were nonevaluable for MRD status, generally because of peripheral blood (PB) contamination (n = 60) or technical failure (n = 40). The median limit of detection (LOD) was 7.4 × 10−6 (range, 6.6 × 10−7 to 1.3 × 10−3). The logarithmic range of the LOD was similar between the ixazomib and placebo arms within each geographic region (supplemental Table 1).
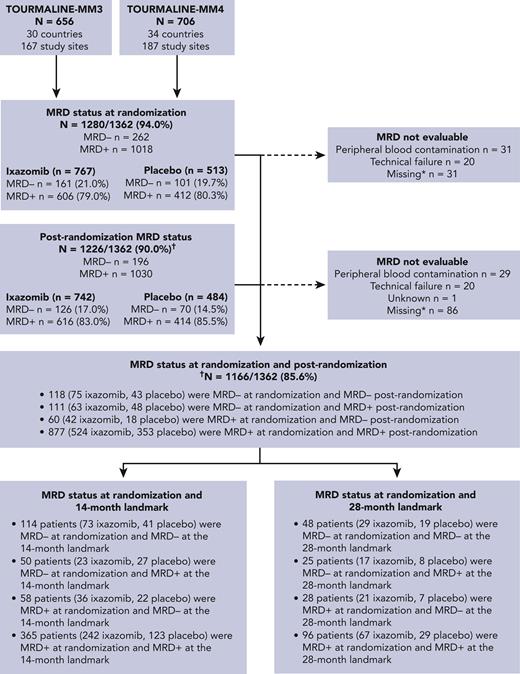
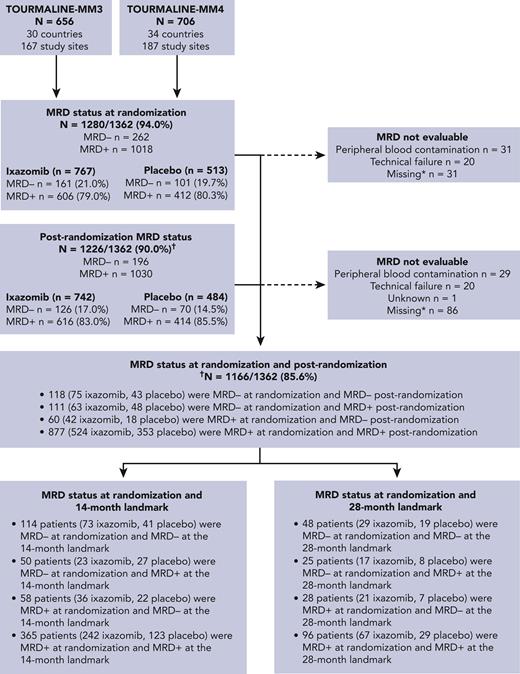
Of the 1362 patients in this analysis, MRD status was available at randomization in 1280, with 82 missing due to PB contamination, technical failure, or absence of sample in CR patients (Figure 1). Details of MRD data imputation (mainly due to PD) are provided in supplemental Table 2. Of these, 262 (20.5%) had undetectable (MRD−) and 1018 (79.9%) detectable (MRD+) MRD. Patient demographics, except age, were generally well balanced between MRD− vs MRD+ patients (Table 1). Of those with MRD− vs MRD+ status at randomization, 90.1% vs 77.0%, were aged <75 years, which was likely a consequence of the greater MRD− rates observed in patients who received ASCT (MM3) vs those who did not (MM4). There were no notable differences between patients who were MRD− and MRD+ at baseline in terms of preinduction ISS stage, cytogenetic risk at diagnosis, or prior treatment (Table 1). However, there was an imbalance in high-risk cytogenetic status between the treatment arms in patients who were MRD− at baseline.
CONSORT-like diagram. Patient disposition according to MRD status throughout the TOURMALINE-MM3 and -MM4 trials. ∗Patients with less than a CR and missing MRD data were imputed as MRD+; ongoing patients in CR and missing MRD data were classified as “missing”. †Post-randomization is at any time point from randomization until end of treatment.
CONSORT-like diagram. Patient disposition according to MRD status throughout the TOURMALINE-MM3 and -MM4 trials. ∗Patients with less than a CR and missing MRD data were imputed as MRD+; ongoing patients in CR and missing MRD data were classified as “missing”. †Post-randomization is at any time point from randomization until end of treatment.
Prognostic impact of MRD status at randomization
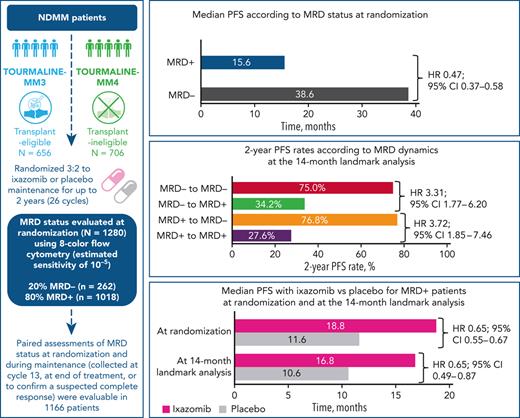
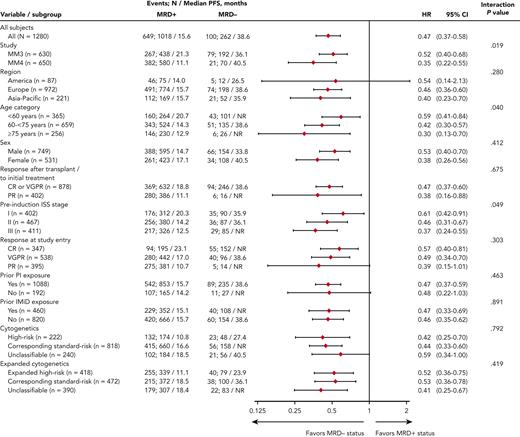
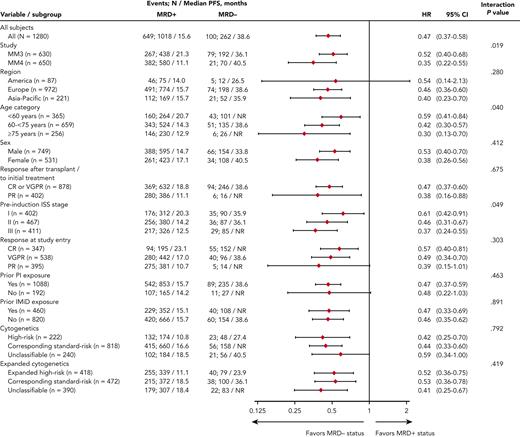
Median PFS from randomization was reached and differed by 23 months between MRD− vs MRD+ patients (Figure 2): 38.6 vs 15.6 months (hazard ratio [HR], 0.47; 95% confidence interval [CI], 0.37-0.58). MRD− status was associated with prolonged PFS vs MRD+ status in nearly all patient subgroups regardless of demographics, disease features at diagnosis including cytogenetic risk, prior treatment, conventional response, or geographic region of MRD testing (Figure 2 and supplemental Figure 2). Noteworthy interactions were observed according to study, age, and ISS stage: MRD negativity in patients enrolled in TOURMALINE-MM4, aged ≥75 years, or with ISS stage III resulted in a greater reduction in the risk of PD or death (Figure 2). In a Cox regression multivariable analysis of PFS, preinduction ISS stage, cytogenetic risk at diagnosis, response at randomization, treatment with ixazomib vs placebo, and MRD status showed independent prognostic value (Table 2).
Univariate analysis of PFS based on IRC assessment. Analysis of the interaction between subgroup variables and MRD status at randomization. NR, not reached.
Univariate analysis of PFS based on IRC assessment. Analysis of the interaction between subgroup variables and MRD status at randomization. NR, not reached.
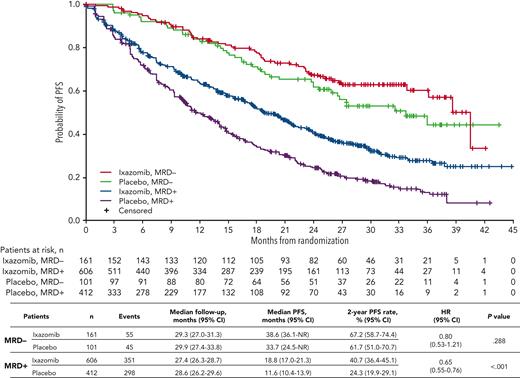
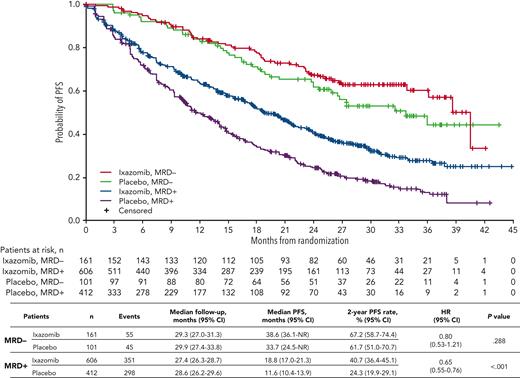
The impact of ixazomib vs placebo on PFS was investigated in patients stratified according to MRD status at randomization. The respective median PFS was 38.6 vs 33.7 months (HR, 0.80; 95% CI, 0.53-1.21; P = .288) in MRD− patients, and 18.8 vs 11.6 months (HR, 0.65; 95% CI, 0.55-0.76; P < .001) in those with MRD+ status (Figure 3). The PFS curves by treatment and MRD status at randomization in each source study are shown in supplemental Figure 3. There was a greater reduction in the risk of PD and/or death with ixazomib vs placebo among patients with MRD+ levels in the 10−5 logarithmic range (HR, 0.45) compared with those with ≥10−4 MRD cells (HR, 0.66) (supplemental Figure 4).
PFS with ixazomib vs placebo according to MRD status at randomization. Kaplan-Meier analysis of PFS for patients with MRD+ or MRD− status at randomization who received ixazomib or placebo in the TOURMALINE-MM3 and -MM4 trials. NR, not reached.
PFS with ixazomib vs placebo according to MRD status at randomization. Kaplan-Meier analysis of PFS for patients with MRD+ or MRD− status at randomization who received ixazomib or placebo in the TOURMALINE-MM3 and -MM4 trials. NR, not reached.
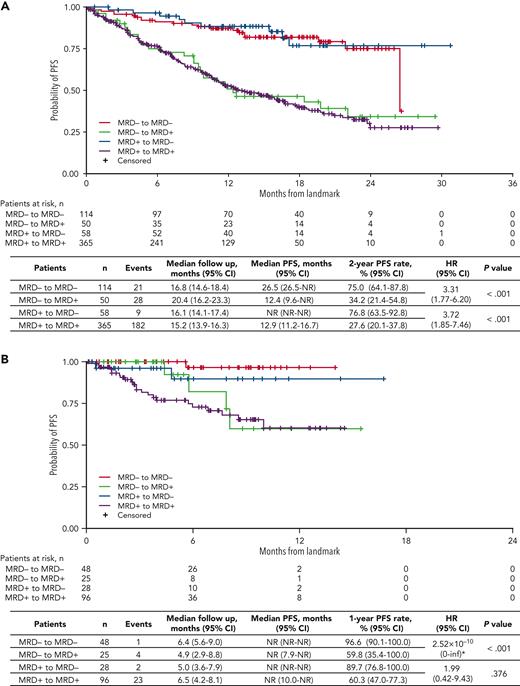
Dynamic MRD status and risk of progression
At any time during ixazomib or placebo administration, MRD status was available in 1226 patients; of these, 196 (16.0%) were MRD− and 1030 (84.0%) MRD+ (Figure 1). MRD dynamics (ie, paired assessments at randomization and during maintenance) were evaluable in 1166 patients: 118 patients (10.1%) showed sustained MRD−, 111 (9.5%) converted from MRD− to MRD+, 60 (5.1%) converted from MRD+ to MRD−, and 877 (75.2%) had persistent MRD+ (Figure 1 and Table 3). Thus, nearly half of MRD− patients at randomization lost their MRD− status in <2 years (n = 111/229 [48.5%]), and a small proportion of MRD+ cases were able to convert into MRD− status during maintenance (n = 60/937 [6.4%]).
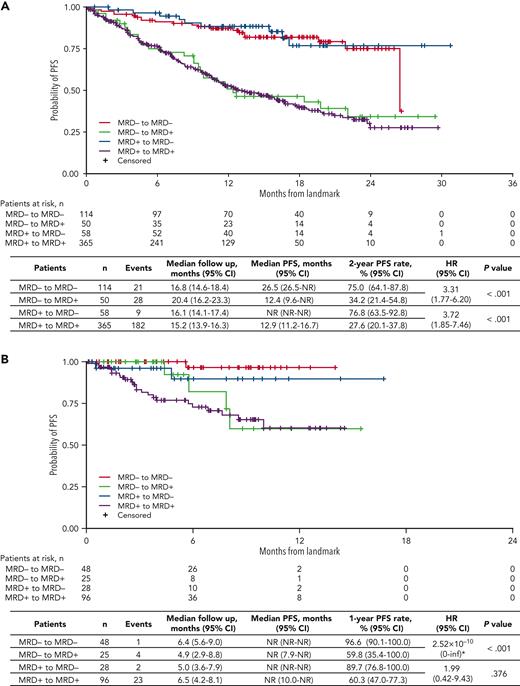
In the 14-month landmark analysis of PFS according to MRD dynamics, 2-year PFS rates were 76.8% (95% CI, 63.5%-92.8%) in patients converting from MRD+ to MRD− status (n = 58) and 75.0% (95% CI, 64.1%-87.8%) in those with sustained undetectable MRD (n = 114) (Figure 4A). In patients who converted from MRD− to MRD+ status (n = 50) and with persistent MRD (n = 365), 2-year PFS rates were 34.2% (95% CI, 21.4%-54.8%) and 27.6% (95% CI, 20.1%-37.8%), respectively. There was an increased risk of PD or death from the time of the landmark in patients who had converted from MRD− to MRD+ status vs those with sustained undetectable MRD (HR, 3.31, 95% CI 1.77-6.20, P < .001), and in those with persistent MRD+ vs patients who had converted from MRD+ to MRD− status (HR 3.72; 95% CI, 1.85-7.46; P < .001). The 28-month landmark analysis of PFS indicated similar findings (Figure 4B). Changes in MRD dynamics according to clinical study are presented in supplemental Table 3.
Landmark analyses of PFS regardless of treatment received. Landmark analysis based on MRD kinetics from randomization to (A) 14 months and (B) 28 months, regardless of ixazomib or placebo treatment in the TOURMALINE-MM3 and -MM4 trials. inf, infinity; NR, not reached. ∗HR, of MRD− to MRD+ vs sustained MRD− unstable due to small event numbers.
Landmark analyses of PFS regardless of treatment received. Landmark analysis based on MRD kinetics from randomization to (A) 14 months and (B) 28 months, regardless of ixazomib or placebo treatment in the TOURMALINE-MM3 and -MM4 trials. inf, infinity; NR, not reached. ∗HR, of MRD− to MRD+ vs sustained MRD− unstable due to small event numbers.
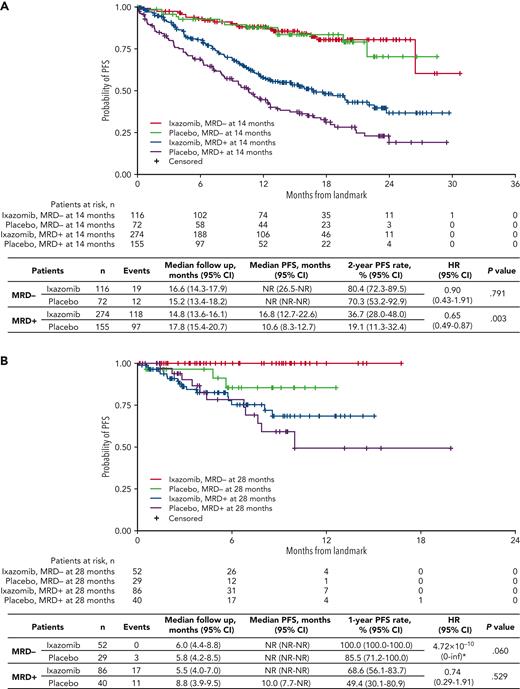
Impact of ixazomib maintenance vs placebo in MRD− and MRD+ patients
Considering the noteworthy modulation in risk of progression according to MRD dynamics, we investigated the impact of ixazomib vs placebo on evolving MRD status. MRD conversions from randomization to the 14-month landmark by treatment group are presented in supplemental Table 4. Patients treated with ixazomib vs placebo showed a borderline statistically significant difference in the rates of sustained MRD negativity (76.0% vs 60.3%, respectively; P = .04). No differences were observed in the rate of conversions from MRD+ to MRD− status.
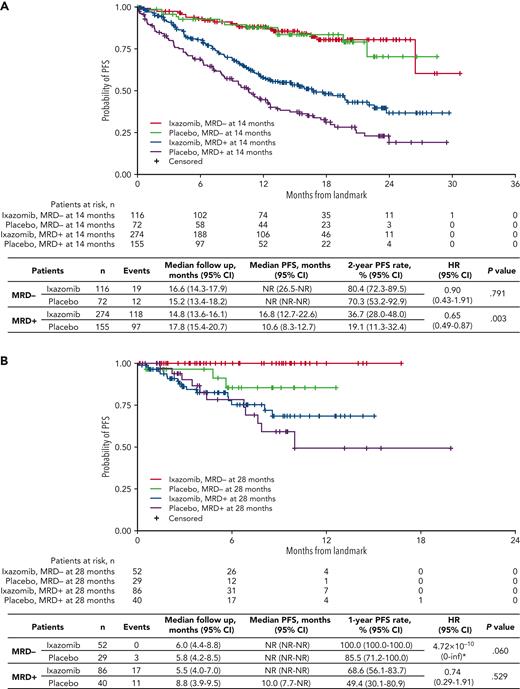
In the 14-month landmark analysis of PFS according to treatment group (Figure 5A), 2-year PFS rates were 36.7% (95% CI, 28.0%-48.0%) vs 19.1% (95% CI, 11.3%-32.4%) in patients who were MRD+ at the 14-month landmark in the ixazomib (n = 274) vs placebo (n = 155) groups, (median PFS 16.8 vs 10.6 months; HR, 0.65; 95% CI, 0.49-0.87; P = .003). Patients who had persistent MRD from randomization to the 14-month landmark (n = 169) were analyzed according to whether their level of MRD had increased or not during that time. With ixazomib vs placebo, the PFS benefit appeared greater in patients whose MRD levels had not increased from randomization to the 14-month analysis (HR, 0.39; 95% CI, 0.22-0.68; P < .001) compared with patients whose MRD levels had increased (HR, 0.67; 95% CI, 0.22-2.07; P = .487) (supplemental Figure 5).
Landmark analyses of PFS with ixazomib vs placebo. Landmark analysis based on MRD status at (A) 14 months and (B) 28 months in patients who received ixazomib vs placebo in the TOURMALINE-MM3 and -MM4 trials. inf, infinity; NR, not reached. ∗HR, of MRD− with ixazomib vs placebo unstable due to small event numbers.
Landmark analyses of PFS with ixazomib vs placebo. Landmark analysis based on MRD status at (A) 14 months and (B) 28 months in patients who received ixazomib vs placebo in the TOURMALINE-MM3 and -MM4 trials. inf, infinity; NR, not reached. ∗HR, of MRD− with ixazomib vs placebo unstable due to small event numbers.
In patients who were MRD− at the landmark, 2-year PFS rates were 80.4% (95% CI, 72.3%-89.5%) vs 70.3% (95% CI, 53.2%-92.9%) in the ixazomib (n = 116) vs placebo (n = 72) groups, (median PFS not reached in either arm; HR, 0.90; 95% CI, 0.43-1.91; P = .791) (Figure 5A). Findings were similar for the 28-month landmark analysis (Figure 5B), though notably, none of the patients with undetectable MRD at the landmark receiving ixazomib had progressed in the short follow-up time.
Discussion
This pooled analysis of the TOURMALINE-MM3 and -MM4 trials includes the largest data set ever reported evaluating the impact of evolving MRD status during active maintenance or placebo (observation) post-induction in transplant-eligible and -ineligible patients with MM. Five main conclusions emerged from this study: (1) MRD status is dynamic and its prognostic value increased considerably with periodic vs single assessment; (2) the favorable prognosis of undetectable MRD was similar if achieved before or during maintenance, and therefore it can become a relevant end point in this setting; (3) patients who lost MRD− status had a poor PFS similar to patients with persistent MRD; (4) treatment with ixazomib vs placebo improved the PFS in patients who were MRD+ at randomization or at the 14-month landmark; and (5) 2-year ixazomib maintenance was not efficacious in patients who were MRD− at these time points.
The subgroup analyses showed that MRD− status was associated with prolonged PFS vs MRD+ status in almost all patient groups analyzed. This finding also applied to patients with standard- vs high-risk cytogenetics, which is consistent with previous reports,6,10 although caveated by imbalances in cytogenetic risk status between treatment groups in MRD− patients and the high number of unclassifiable patients. Thus, MRD status at randomization was an independent prognostic factor for PFS, together with other disease- and treatment-related covariates. That notwithstanding, and acknowledging differences between study designs and populations, the median PFS of MRD− patients (∼3 years from randomization) appeared shorter than in studies using less sensitive MRD assessment methods. Lahuerta et al reported a median PFS of ∼5 years for 259 MRD− patients according to multiparameter flow cytometry at a sensitivity level of 10−4 to 10−5,16 and Mohan et al reported a median PFS exceeding 10 years for 344 MRD− patients according to next-generation flow cytometry at a sensitivity of 10−5.17 Furthermore, the reduction in risk of PD or death associated with MRD− status was lower than that reported in a large meta-analysis of 8098 patients (53% vs 67%) by Munshi et al.2 These findings highlight the need to analyze MRD dynamics over time to shed light onto the unexpectedly poor median PFS in patients with MRD− status at randomization in our study, which included both transplant-eligible and transplant-ineligible patients randomized to fixed-duration or no maintenance.
A recent analysis of 2 trials showed that the PFS of MRD− patients enrolled in ALCYONE (clinicaltrials.gov, number NCT02195479) appeared to be inferior to those in MAIA (clinicaltrials.gov, number NCT02252172), particularly if MRD− status was not sustained for at least 12 months.4 Thus, compared with a doublet or triplet as used until PD in MAIA, single-agent daratumumab or observation after induction in ALCYONE may have contributed to shorter PFS upon MRD reappearance, in line with our data of single-agent ixazomib or placebo. In the present pooled analysis of TOURMALINE-MM3 and -MM4, about one-half of the patients converted from MRD− to MRD+ in a relatively short follow-up time, this proportion being lower in the ixazomib vs the placebo arm. Furthermore, our results show that a change from MRD− status at randomization to MRD+ status at 14 months was associated with an increased risk of progression, with a median PFS from the landmark of ∼1 year. This information is relevant to future trials exploring early intervention upon MRD reappearance.18
Owing to the limited number of studies that have reported MRD dynamics based on serial assessments, the clinical implications of MRD status over time have not been clearly defined. Here we support previous observations from the IFM 2009,6 EMN02/HO95,8 and Myeloma XI10 trials, by showing that patients converting from MRD+ to MRD− status had superimposable PFS to that of cases with sustained undetectable MRD at the 14- and 28-month landmarks. Because the PFS in these patients was notably longer than in patients with persistent MRD, the consideration of MRD negativity as an end point of treatment could be extrapolated to the maintenance setting.
Whether continuous maintenance therapy is equally beneficial for all patients, or if treatment-free intervals can be safely offered in some cases, is a matter of debate. Our results, with the caveat of progressively shorter follow-up, suggest that the risk of progression was lower in MRD− patients at the 28- vs the 14-month landmark vs randomization, thus reproducing the previous observations made by Gu et al and Diamond et al.9,19 A much longer follow-up is required to uncover the proportion of patients at risk of relapse and if the risk is linear throughout the years or if a plateau is achievable.
In contrast to the uncertain clinical outcomes of an MRD− result, persistence of MRD is, except for a few patients,20 almost always associated with PD. Therefore, it may be more reasonable to act on MRD+ status rather than MRD− status, with the persistence of MRD resulting in treatment intensification with the aim of improving dismal outcomes. To our knowledge, this is the first study showing that, in patients with MRD+ status before maintenance, treatment with ixazomib vs placebo improved PFS. Findings were similar at the 14-month but not at the 28-month landmark, when treatment was ceased in TOURMALINE-MM3 and -MM4. Altogether, these results stress that stopping maintenance therapy in MRD+ patients is associated with imminent risk of relapse.
Another interesting observation from this study was that a greater reduction in the risk of PD or death with ixazomib vs placebo was observed in patients with MRD+ in the logarithmic range of 10−5 compared with those with ≥10−4 MRD cells. In addition, patients with persistent MRD whose MRD levels had not increased from randomization to the 14-month landmark analysis had a greater reduction in risk of PD or death with ixazomib vs placebo compared with patients whose MRD levels increased from randomization to the 14-month landmark. These findings, together with the lack of differences in the rate of conversions from MRD+ to MRD− status with ixazomib vs placebo, suggest that rather than eradicating MRD, single-agent treatment was able to control the size of the clone for longer periods. Therefore, it can be argued that more intensive regimens are needed to abrogate the poor prognosis of higher levels of MRD (eg, 10−4) before and during maintenance. These results highlight the importance of regular monitoring of not just MRD status, but also MRD levels, to evaluate efficacy and guide therapeutic decisions.
Next-generation flow (NGF) and next-generation sequencing (NGS), which enable MRD evaluation at sensitivity levels of 10−6, are now routinely used in trials.6,7,12,21,22 However, NGF was not widely available during the design of the TOURMALINE-MM3 and -MM4 MRD studies, and a diagnostic sample that is needed for NGS was not systematically stored. Therefore, a flow cytometry assay was specifically developed and validated to assess MRD with an estimated sensitivity of 4 × 10−6. This was largely achieved, with 99% of patients assessed at a sensitivity of ≥10−5. Accordingly, the present study demonstrated the feasibility of routine MRD evaluation with the use of flow cytometry in large trials. Interestingly, the geographic region where lower sensitivity was achieved (Americas) produced inferior results regarding the impact of MRD negativity on the reduction in risk of PD or death (HR, 0.54 vs 0.46 and in Europe and 0.40 in Asia-Pacific). These results offer a pragmatic confirmation that evaluation of MRD status at a higher degree of sensitivity is associated with a better prediction of patient outcomes.2,6,7,23,24
In addition to sensitivity, the accuracy of MRD status also benefits from serial assessment, as shown here and elsewhere.9,13,19 Therefore, using a minimally invasive and nonbiopsy approach where MRD can be viewed more frequently and more broadly, such as mass spectrometry, positron emission tomography (PET), or computed tomography (CT), might be a valuable adjunct to NGS or NGF as part of a regular follow-up schema.25-28 MRD assessment in PB using of NGS or NGF has been recently investigated, with promising preliminary results.29 Despite the lower sensitivity of PB vs BM, detection of MRD in the former was significantly associated with dismal PFS.29,30 Therefore, it could be envisioned that serial MRD assessments using either NGS or NGF in BM, spaced for a certain amount of time (eg, 1 or 2 years), could be intercalated with more frequent PB evaluations (eg, every 6 months).
Optimal balance between treatment efficacy and toxicity is of utmost importance to preserve quality of life. According to our results, for patients who do not achieve MRD− status or who convert from MRD− to MRD+ status, it should be noted that although prolonged therapy significantly improved PFS, the benefit was limited to certain cases (potentially those converting from MRD+ to MRD−). Therefore, the role of MRD in treatment decisions remains to be established and is being investigated in ongoing randomized studies evaluating MRD-directed therapy31 (GEM2014MAIN [NCT02406144], RADAR [UK-MRA Myeloma XV] [clinicaltrialsregister.eu, number 2019-001258-25], NCT03742297, DRAMMATIC [NCT04071457],32 Perseus [NCT03710603], KRdvsRd [NCT04096066]). Importantly, our results highlight that MRD-directed therapy should rely on MRD dynamics rather than MRD status assessed at a single time point. Thus, our study sets the stage for serial MRD monitoring during maintenance or observation in clinical trials.
Acknowledgments
The authors thank the patients and their families, as well as the physicians, nurses, study coordinators, and research staff, for participation in the trial.
This work was supported by Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. Editorial support was provided by Victoria Enwemadu of Takeda Pharmaceuticals USA, Inc., Lexington, MA; and medical writing support for the development of this manuscript, under the direction of the authors, was provided by Philippa Lloyd and Steve Hill of Ashfield MedComms, an Inizio Company, funded by Takeda Pharmaceuticals USA, Inc., Lexington, MA and complied with good publication practice (GPP) guidelines (DeTora LM et al, Ann Intern Med 2022;175:1298-1304).
Authorship
Contribution: B.P., B.W., M.A.D., C.-K.M., R.L., and R.T. designed the study; B.P., I.M., M.A.D., F.G., C.-K.M., S.Z., I.Š., R.T., M.-V.M., N.G., M.C., C.R.H., and W.F. performed study investigations; M.A.D., F.G., C.-K.M., S.Z., I.Š., R.T., M.-V.M., N.G., M.C., C.R.H., and W.F. enrolled patients; B.P., I.M., A.B.D., C.-K.M., M.A.D., and J.E. collected data; B.P., I.M., C.L., A.B.D., J.E., B.W., A.V., K.S., M.A.D., and C.-K.M. analyzed data; B.P., I.M., K.S., C.L., R.L., A.B.D., J.E., B.W., A.V., and M.A.D. interpreted data; all authors prepared, reviewed, revised, and provided final approval of the manuscript.
Conflict-of-interest disclosure: B.P. reports consultancy fees for BMS-Celgene, GSK, Janssen, Sanofi, and Takeda, research funding from BMS-Celgene, GSK, Roche, and Sanofi, and honoraria from Adaptive, Amgen, Becton Dickinson, BMS-Celgene, GSK, Janssen, Sanofi, and Roche. M.A.D. reports honoraria from participation in advisory boards from Amgen, BMS, Janssen, Takeda, and Beigene. F.G. reports honoraria from Janssen, Takeda, BMS, Celgene, AbbVie, Roche, Pfizer, Sanofi, Adaptive, and Oncopeptides. S.Z. reports research funding from Takeda and Janssen and honoraria from Celgene-BMS, Janssen, Oncopeptides, Sanofi, and Takeda. I.S. reports honoraria from Amgen, Janssen, Sanofi, BMS, GSK, Karyopharm, and Takeda. R.T. reports consultancy fees from Takeda, GSK, Janssen, and BMS/Celgene, research funding from Janssen, and honoraria from BMS/Celgene, Janssen, Amgen, Gilead, and Novartis. M.-V.M. reports honoraria from AbbVie, Adaptive, Amgen, Bluebird-bio, Celgene-BMS, Genentech/Roche, GSK, Karyopharm, Janssen, Oncopeptides, Pfizer, Sanofi, and Takeda. N.G. reports research funding from Celgene, Takeda, and Janssen and honoraria from Amgen, BMS, Celgene, Takeda, and Janssen. M.C. reports honoraria from Janssen, Celgene/BMS, Sanofi, Takeda, Amgen, MundiPharma, AbbVie, Adaptive, and GSK and served on a speakers’ bureau for Janssen and Celgene/BMS. C.R.H. reports honoraria from Roche, Astra Zeneca, Takeda, Novartis, Janssen, and Pfizer and membership on an advisory board/committee for Fundación Cancer Vida. K.S. reports employment with Takeda. A.V. reports employment with Takeda and holds stocks in Takeda. C.L. reports employment with Takeda. B.W. reports employment with Takeda. J.E. reports employment with Takeda. R.L. reports employment with Takeda. A.B.D. reports employment with and holds stock in Takeda. The remaining authors declare no competing financial interests.
Correspondence: Bruno Paiva, Clinica Universidad de Navarra, Centro de Investigación Médica Aplicada (CIMA), Av de Pío XII, 36, 31008 Pamplona, Spain; e-mail: bpaiva@unav.es.
References
Author notes
The data sets, including the redacted study protocols, redacted statistical analysis plans, and individual participant data supporting the results of the completed study will be made available after the publication of the final study results within 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after its deidentification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.