Key Points
The novel combination of tafasitamab ± lenalidomide + R-CHOP showed signs of efficacy in patients with untreated DLBCL, with no new safety signals.
The results, including a post hoc analysis in patients with high-risk disease (IPI 3-5), support the ongoing phase 3 frontMIND trial.
Abstract
Anti-CD19 immunotherapy tafasitamab is used in combination with lenalidomide in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) who are ineligible for autologous stem cell transplant. Open-label, phase 1b, First-MIND study assessed safety and preliminary efficacy of tafasitamab + R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) ± lenalidomide as first-line therapy in patients with DLBCL. From December 2019 to August 2020, 83 adults with untreated DLBCL (International Prognostic Index 2-5) were screened and 66 were randomly assigned (33 per arm) to R-CHOP-tafasitamab (arm T) or R-CHOP-tafasitamab-lenalidomide (arm T/L) for 6 cycles. Primary end point was safety; secondary end points included end-of-treatment (EoT) overall response rate (ORR) and complete response (CR) rate. All patients had ≥1 treatment-emergent adverse event, mostly grade 1 or 2. Grade ≥3 neutropenia and thrombocytopenia occurred, respectively, in 57.6% and 12.1% (arm T) and 84.8% and 36.4% (arm T/L) of patients. Nonhematologic toxicities occurred at similar rates among arms. R-CHOP mean relative dose intensity was ≥89% in both arms. EoT ORR was 75.8% (CR 72.7%) in arm T and 81.8% (CR 66.7%) in arm T/L; best ORR across visits was 90.0% and 93.9%. Eighteen-month duration of response and of CR rates were 72.7% and 74.5% (arm T) and 78.7% and 86.5% (arm T/L); 24-month progression-free and overall survival rates were 72.7% and 90.3% (arm T) and 76.8% and 93.8% (arm T/L). Manageable safety and promising signals of efficacy were observed in both arms. Potential benefit of adding tafasitamab + lenalidomide to R-CHOP is being investigated in phase 3 frontMIND (NCT04824092). This study is registered at www.clinicaltrials.gov as #NCT04134936.
Introduction
Diffuse large B-cell lymphoma (DLBCL), the most common non-Hodgkin lymphoma (NHL) subtype, accounts for 25% to 45% of all NHL cases worldwide.1 Standard first-line treatment is 6 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), but 30% to 40% of patients experience relapsed or refractory (R/R) disease.2 R-CHOP failure rates for patients with an International Prognostic Index (IPI) score of 3 to 5 (higher-risk disease) approach 50%.3,4
Several phase 3 trials have attempted to improve outcomes with R-CHOP by adding supplementary agents, such as ibrutinib (PHOENIX5), lenalidomide (ROBUST6), polatuzumab vedotin (POLARIX7), or bortezomib (REMoDL-B8); however, all failed to improve overall survival (OS). Only POLARIX7 demonstrated improved progression-free survival (PFS), and on the basis of the results of this trial, polatuzumab vedotin with R-CHP (rituximab, cyclophosphamide, doxorubicin, and prednisone) is now approved for use in several countries. Nevertheless, an unmet need remains to improve the current standard of care for patients with treatment-naïve DLBCL, particularly those with higher-risk disease.
Lenalidomide, an immunomodulatory drug with antineoplastic activity, has shown promise for patients with NHL who were heavily pretreated, including a retrospective study conducted in Italy,9 but the results of adding lenalidomide to R-CHOP (R2-CHOP) varied.10 In the randomized phase 2 Eastern Cooperative Oncology Group (ECOG)-ACRIN trial E1412, adding lenalidomide (25 mg per day, days 1-10 of each 21-day cycle) to R-CHOP improved PFS (73% vs 62%; P = .03) and OS (83% vs 75%; P = .05) at 3 years, but the results of this signal-seeking study were not considered practice-changing without corroborative evidence.11 In the phase 3 ROBUST study in patients who did not receive treatment with activated B-cell (ABC) subtype of DLBCL6 (a high-risk subtype with reported 5-year OS of ∼50% vs ∼80% in patients with the germinal center B-cell [GCB] subtype [P = .001]),12 the addition of lenalidomide (15 mg/day, days 1-14 per cycle) to R-CHOP did not improve PFS or OS.6 However, there was a trend toward PFS benefit in patients with IPI ≥3.6 In both studies, grade ≥3 adverse events (AEs) were higher in patients receiving R2-CHOP than those receiving R-CHOP alone.6,11
Tafasitamab, a humanized monoclonal antibody, targets CD19 and functions as an immunotherapy through direct cytotoxicity, antibody-dependent cellular cytotoxicity (ADCC), and antibody-dependent cellular phagocytosis (ADCP).13 It has a unique Fc domain that enhances its affinity to natural killer (NK) cells and macrophages, which leads to increased ADCC and ADCP. In the phase 2 L-MIND trial, in 80 patients with R/R DLBCL who were ineligible for autologous stem cell transplant, the combination of tafasitamab and lenalidomide achieved an overall response rate (ORR) at 5-year follow-up of 57.5%, complete response (CR) rate of 41.3%, median duration of response not reached, median PFS of 11.6 months, and median OS of 33.5 months;14 previously, the primary data were the basis for accelerated approval in the United States (July 2020) and conditional marketing authorization in Europe (August 2021) in this second-line setting.15,16
We hypothesized that the addition of tafasitamab ± lenalidomide to R-CHOP may improve first-line outcomes. Given the availability of data on R2-CHOP in first line and tafasitamab + lenalidomide in second and later lines discussed above, we were able for the first time to design a study testing the addition of 2 agents to standard-of-care R-CHOP as first-line therapy. Thus, the First-MIND study evaluated the safety and efficacy of tafasitamab ± lenalidomide with R-CHOP in patients with previously untreated DLBCL.
Methods
Study design and participants
The phase 1b, open-label, multicenter, randomized First-MIND study (NCT04134936)17 was conducted at 53 sites in North America and Europe. Eligible patients were aged ≥18 years with newly diagnosed, previously untreated, histologically confirmed DLBCL not otherwise specified; had at least one measurable lesion confirmed by positron emission tomography (PET)–positive at the time of randomization; ECOG performance status (ECOG-PS) from 0 to 2; IPI status from 2 to 5; and were candidates for R-CHOP as first-line therapy (Figure 1). Patients with known double- or triple-hit lymphoma, transformed NHL, and evidence of composite lymphoma were excluded in order to minimize the heterogeneity of the patient population being studied.
Study design. ∗In the lenalidomide arm, venous thromboembolism prophylaxis with either low-molecular weight heparins or aspirin is mandatory (according to institutional guidelines). †Rituximab (375 mg/m2) and CHOP chemotherapy included cyclophosphamide (750 mg/m2 IV), doxorubicin (50 mg/m2 IV), and vincristine (1.4 mg/m2 [maximum dose = 2 mg] IV) on day 1 of every 21-day cycle and prednisone/prednisolone (100 mg/day PO) on days 1 to 5. The day 1 steroid dose being part of CHOP (100 mg prednisone/prednisolone, or equivalent, PO or IV) could be used as a further component of premedication before tafasitamab infusion. L, lenalidomide; NOS, not otherwise specified; PO, orally; R, randomized; T, tafasitamab.
Study design. ∗In the lenalidomide arm, venous thromboembolism prophylaxis with either low-molecular weight heparins or aspirin is mandatory (according to institutional guidelines). †Rituximab (375 mg/m2) and CHOP chemotherapy included cyclophosphamide (750 mg/m2 IV), doxorubicin (50 mg/m2 IV), and vincristine (1.4 mg/m2 [maximum dose = 2 mg] IV) on day 1 of every 21-day cycle and prednisone/prednisolone (100 mg/day PO) on days 1 to 5. The day 1 steroid dose being part of CHOP (100 mg prednisone/prednisolone, or equivalent, PO or IV) could be used as a further component of premedication before tafasitamab infusion. L, lenalidomide; NOS, not otherwise specified; PO, orally; R, randomized; T, tafasitamab.
Patients were randomly assigned (without stratification) to either R-CHOP + tafasitamab or R-CHOP + tafasitamab + lenalidomide in a 1:1 ratio through interactive response technology. This study was approved by the Institutional Review Board and was conducted in accordance with the International Conference on Harmonization good clinical practice guidelines and the Declaration of Helsinki. All the patients provided written informed consent.
Procedures
Treatment comprised R-CHOP + tafasitamab (arm T) or R-CHOP + tafasitamab + lenalidomide (arm T/L) for up to six 21-day cycles. R-CHOP consisted of rituximab 375 mg/m2 administered IV on day 1, cyclophosphamide 750 mg/m2 IV on day 1, doxorubicin 50 mg/m2 IV on day 1, vincristine 1.4 mg/m2 IV on day 1, and prednisone 100 mg orally (or equivalent), once daily, on days from 1 to 5. Tafasitamab was administered at a dose of 12 mg/kg IV on days 1, 8, and 15 of each 21-day cycle as per the label and the protocol.15,16 Lenalidomide was administered orally at a starting dose of 25 mg daily (the dose used in the positive ECOG-ACRIN trial E1412 of R2-CHOP in first line,11 and approved in combination with tafasitamab in patients with R/R DLBCL ineligible for ASCT15,16) on days 1 to 10 of each 21-day cycle. Lenalidomide dose reductions were permitted, if required, to a minimum of 10 mg.
Granulocyte colony–stimulating factor (G-CSF) or pegylated G-CSF was mandatory for the prophylaxis of neutropenia and was administered as per institutional guidelines. Owing to increased risk of thrombosis in patients treated with lenalidomide, venous thromboembolism prophylaxis with aspirin or low-molecular weight heparin was mandatory for patients receiving lenalidomide. Central nervous system prophylaxis was permitted, according to institutional practice and had to be preplanned before randomization. Preplanned local radiotherapy could be administered to initial sites of bulky disease or extranodal disease according to institutional guidelines (no definition of bulky disease was specified as per protocol) but only after the last treatment cycle and after the end of treatment (EoT) tumor assessment by PET/computed tomography (CT) or PET/magnetic resonance imaging (MRI). Patients were followed up every 3 months from the day of EoT or early study treatment discontinuation visit to 18 months after EoT visit.
A mandatory retrospective central pathology review was performed to confirm diagnosis of DLBCL. Tumor assessment was performed using CT and PET scans before treatment, midtreatment (cycle 3, day 18), and at EoT visit (4-8 weeks after the last study treatment). Responses were assessed by investigators using Lugano 2014 criteria.18 During follow-up, clinical evaluation and CT scans were performed every 3 and 6 months, respectively, until disease progression/relapse or final study completion. Bone marrow assessment was not mandated in patients who underwent PET/CT or PET/MRI as per Lugano 2014 criteria; decision was made according to local guidelines.
Outcomes
The primary end point was incidence and severity of treatment-emergent AEs (TEAEs) from the first dose of study treatment until 30 days after day 21 of the last treatment cycle. TEAEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA, version 24.0) system organ class and preferred terms; toxicity was determined according to the National Cancer Institute Common Terminology Criteria for AEs (NCI-CTCAE) version 5.0. AEs of special interest were tumor lysis syndrome, second primary malignancies, infusion-related reactions (IRR), allergic reactions to study drug grade ≥3, cytokine release syndrome, hepatitis B reactivation, and progressive multifocal leukoencephalopathy. Key secondary end points included ORR, PET–negative CR rate, and the other secondary end points included partial response (PR) rate at the EoT, PFS, and OS at 12 and 24 months. Additional end points were duration of response (DoR) and duration of complete response (DoCR).
Exploratory analyses
Blood and tumor samples were collected before treatment, and blood samples were collected throughout the study for assessment of exploratory biomarkers, including B-, T- and NK-cell counts in peripheral blood and cell of origin (COO) (further details on COO are provided in supplemental Information; available on the Blood website). Circulating tumor DNA (ctDNA) quantification was also undertaken to monitor residual disease burden (minimal residual disease [MRD]) using immunoglobulin gene next-generation sequencing in cell-free DNA extracted from plasma. MRD data from this study are being analyzed with other datasets and will be reported separately.
Post hoc exploratory analyses were conducted to assess outcomes in patients with high-intermediate/high-risk disease (IPI score 3-5), partly to provide a reference point for an ongoing randomized phase 3 clinical trial in this subpopulation.19
Statistical analyses
Because this was a phase 1b study primarily conducted to explore safety end points, no formal hypothesis testing was performed for this trial, and the study was not powered to establish efficacy. The rationale for the sample size selection is included in the supplemental Information. Primary and secondary end points were analyzed using descriptive statistics. The full analysis set (FAS) comprised all patients who were randomized to either of the study arms. Efficacy analysis was performed on the FAS. The safety analysis set comprised all patients who received at least one dose of study drug (tafasitamab ± lenalidomide). This study is registered with ClinicalTrials.gov (identifier: NCT04134936).17 Final analysis was performed after the last patient completed their end-of-study visit (18-months after the EoT visit).
Results
Patients
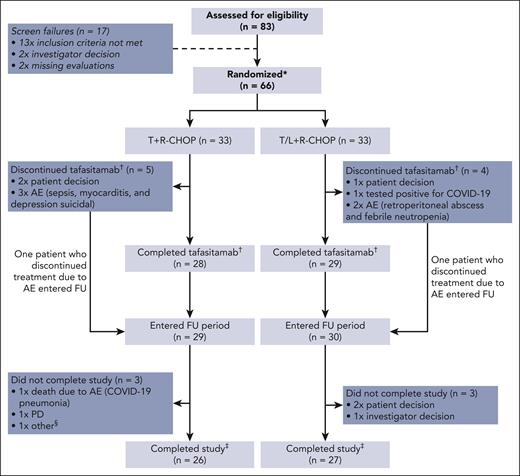
From December 2019 to August 2020, 83 patients were screened. Seventeen patients were excluded during screening; reasons for exclusion were “not meeting inclusion criteria” (n = 13), “investigator’s decision” (n = 2), and “other” (missing baseline evaluations, n = 2) (Figure 2). The remaining 66 patients were finally enrolled in the study (33 in each arm) and received at least 1 dose of either study medication and were evaluated for safety and efficacy (ie, the FAS and safety analysis set were identical). The primary efficacy analysis included ORR at EoT, the final analysis for DoR, DoCR, and safety at 18 months, and the final database lock was on 24 February 2023 to include 24-month data for PFS and OS.
Patient disposition. Notably, also some patients with PD at EoT have entered the FU period. ∗All the patients randomized were included in efficacy analysis (FAS) and safety analysis. †Discontinuations of other study components not shown. ‡Completed study: all FU visits completed. §PD and participation in another clinical trial. AE, adverse event; COVID-19, coronavirus disease 2019; FU, follow-up; PD, progressive disease.
Patient disposition. Notably, also some patients with PD at EoT have entered the FU period. ∗All the patients randomized were included in efficacy analysis (FAS) and safety analysis. †Discontinuations of other study components not shown. ‡Completed study: all FU visits completed. §PD and participation in another clinical trial. AE, adverse event; COVID-19, coronavirus disease 2019; FU, follow-up; PD, progressive disease.
Baseline characteristics were balanced between arm T (R-CHOP + tafasitamab) and arm T/L (R-CHOP + tafasitamab + lenalidomide) (Table 1). The median age of enrolled patients was 64.5 years (range, 20-86); 65% of patients were aged >60 years, and 58% were female. At baseline, patients had a high degree of adverse prognostic factors, such as Ann Arbor stage IV disease (71%), Ann Arbor stage III or IV (94%), and bulky disease (44%). Overall, 36%, 44%, and 20% of patients had IPI 2, 3, and 4 to 5 risk scores, respectively, and 56% had COO of GCB vs 36% non-GCB (missing data, 8%) as assessed centrally (more details on biomarker COO testing are included in the supplemental Information).
Safety
No unexpected safety signals were observed during the safety run-in phase (n = 12 per arm). Hence, the study continued to enroll 21 additional patients in each arm for the main phase.
Overall, 26 (78.8%) patients in arm T and 27 (81.8%) patients in arm T/L completed the study treatment (FAS); 7 patients in arm T and 6 patients in arm T/L discontinued owing to patient withdrawal, AEs, and diagnosis of COVID-19 (Figure 2). Twenty-eight of 33 (84.8%) patients in arm T and 29 of 33 (87.9%) patients in arm T/L completed all 6 cycles of tafasitamab treatment. Lenalidomide treatment was completed in 27 (81.8%) patients in arm T/L.
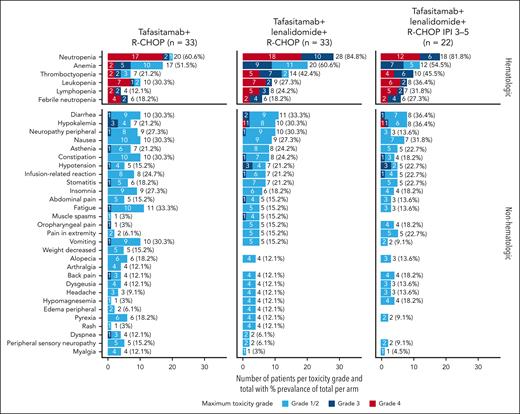
At least 1 TEAE occurred in all 66 patients. The most frequent hematologic and nonhematologic TEAEs occurring in at least 10% of the patients in either arm are summarized in Figure 3. Overall, 86% patients had at least 1 grade ≥3 TEAE (81.8% patients in arm T and 90.9% in arm T/L). The majority of TEAEs were grade 1 to 2 (422/590 events [72%] in arm T and 420/658 events [64%] in arm T/L). Two (6.1%) patients in arm T (owing to sepsis and cardiac disorder) and 1 (3.0%) patient in arm T/L (owing to retroperitoneal abscess) discontinued study treatment owing to AEs. The remaining 3 patients withdrew consent (n = 2 in arm T and n = 1 in arm T/L). Six (9.1%) patients permanently discontinued tafasitamab (3 [9.1%] in each arm: arm T, 1 case each of sepsis, myocarditis, and depression suicidal; arm T/L, 1 case each of COVID-19 pneumonia [this study was conducted during the main COVID-19 pandemic period], retroperitoneal abscess, and febrile neutropenia); and 5 (15.2%) patients discontinued lenalidomide in arm T/L (1 case each of COVID-19 pneumonia, retroperitoneal abscess, and neutropenia and 2 cases of pulmonary embolism [both patients received aspirin or low-molecular weight heparin as venous thromboembolism prophylaxis]). No second primary malignancies (TEAEs of special interest for lenalidomide) were reported. There was 1 case of tumor lysis syndrome in arm T.
Most frequent hematologic and nonhematologic TEAEs occurring in ≥10% of patients in either study arm.
Most frequent hematologic and nonhematologic TEAEs occurring in ≥10% of patients in either study arm.
Serious TEAEs occurred in 31 of 66 (47.0%) patients (n = 14 in arm T and n = 17 in arm T/L), as summarized in supplemental Table 1. There were 3 cases of neurologic serious TEAEs, all in arm T/L including 1 generalized tonic-clonic seizure (grade 4) and 2 cases of syncope (both grade 3). Six deaths occurred, including 1 death owing to progression after EoT (in arm T) and 5 deaths owing to AEs; 1 before (sepsis, in arm T) and 4 after EoT (arm T, 1 case each of urosepsis and COVID-19 pneumonia; arm T/L and 2 cases of COVID-19 pneumonia).
The most commonly occurring hematologic TEAEs (all grade) were neutropenia in 72.7% patients (n = 48/66), anemia in 56.1% (n = 37/66), thrombocytopenia in 31.8% (n = 21/66), and leukopenia in 28.8% (n = 19/66). Grade ≥3 neutropenia occurred in 57.6% (n = 19/33) and 84.8% (n = 28/33) patients in arm T and arm T/L, respectively. Grade ≥3 thrombocytopenia occurred in 12.1% (n = 4/33) patients in arm T, and 36.4% (n = 12/33) patients in arm T/L (Figure 3). Platelet transfusions were required in 9.1% (n = 3/33) of the patients in arm T, and 21.2% (n = 7/33) patients in arm T/L. Despite a numerical increase in the incidence of grade ≥3 neutropenia in arm T/L, the rate of grade ≥3 febrile neutropenia was 6 (18.2%) patients in each arm. In arm T, 4 patients with febrile neutropenia recovered within 14 days; 1 patient recovered within 16 days, and febrile neutropenia was ongoing at the time of data cutoff for 1 patient. In arm T/L, all 6 patients with febrile neutropenia recovered within 14 days.
The most frequently occurring nonhematologic TEAEs (all grade) belonged to gastrointestinal disorder (71.2%), nervous system disorder (62.1%, including only 1 grade 3 event [peripheral neuropathy] in both arms), general disorder and administration site condition (60.6%), infections and infestations (51.5%), and musculoskeletal and connective tissue disorder (45.5%). No patient had a grade ≥3 IRR to either rituximab or tafasitamab in arm T; a grade 3 IRR to rituximab occurred in 1 patient (3.0%) in arm T/L. There were 37 events of infection or infestation (any grade) in 16 patients (48.5%) in arm T and 32 events in 18 patients (54.5%) in arm T/L. Many types of infection were reported, with none predominating. Severe infections were reported in 4 patients (12.1%) in arm T and 3 patients (9.1%) in arm T/L. Grade ≥3 treatment-emergent infection and/or infestation occurred in 7 patients (21.2%) in arm T and 9 patients (27.3%) in arm T/L; at baseline, 1 case of cytomegalovirus infection and 1 of urinary tract infection had been reported in arm T/L. No grade >3 subcutaneous reactions occurred; grade 1 to 2 subcutaneous reactions occurred in 8 (24.2%) of 33 patients in arm T, and in 13 (39.4%) of 33 patients in arm T/L, with no grade 3 events.
The mean average relative dose intensity (ARDI) of R-CHOP was 89.7 ± 24.2% in arm T and 94.0 ± 20.4% in arm T/L, indicating that addition of tafasitamab or tafasitamab + lenalidomide did not substantially affect ARDI of R-CHOP (supplemental Table 2; supplemental Figures 1 and 2). The mean ARDI of lenalidomide was 78.8 ± 28.0% (mean duration of exposure for lenalidomide: 48.8 days of 60 days maximum). The mean ARDI for tafasitamab was 96.5 ± 14.5% in arm T and 97.1 ± 12.7% in arm T/L (mean duration of exposure for tafasitamab: 112.9 days in arm T and 117.3 days in arm T/L). As per protocol, primary neutropenia prophylaxis with G-CSF or pegylated G-CSF was mandatory and was prescribed to all patients as per institutional guidelines (supplemental Table 3).
Tafasitamab interruption after at least 1 TEAE occurred in 9 patients (27.3%) in arm T and 7 patients (21.2%) in arm T/L. Tafasitamab dosing was interrupted in 4 patients (6.1%) during cycle 1 owing to IRRs; 2 patients in each arm, all completed the dose. All occurrences of IRR occurred during the first infusion; no interruptions occurred during later infusions. Interruption of rituximab occurred in 4 patients (12.1%) in each arm during infusion; all completed the dose. Six patients (18.2%) received at least one dose reduction of lenalidomide, owing to hematologic and nonhematologic toxicities (3 patients [9.1%] each) and other reasons (dosing error; 2 patients [6.1%]). Twenty-two patients (66.7%) skipped at least 1 lenalidomide administration in arm T/L. At least 1 dose of R-CHOP (any component) was delayed in 18 of 66 patients (27.3%) owing to AEs; 11 (33.3%) in arm T and 7 (21.2%) in arm T/L.
Efficacy
The ORR at the EoT visit was 75.8% (25/33) and 81.8% (27/33) in arm T and arm T/L, with CR of 72.7% (24/33) and 66.7% (22/33), respectively. At the final analysis (data cutoff: 10 August 2022), the best ORR (proportion of patients with at least 1 confirmed CR or PR during the study) was 90.9% (CR rate, 87.9%; PR rate, 3.0%) in arm T and 93.9% (CR rate, 75.8%; PR rate, 18.2%) in arm T/L.
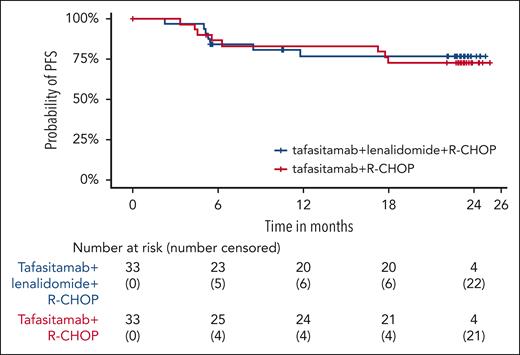
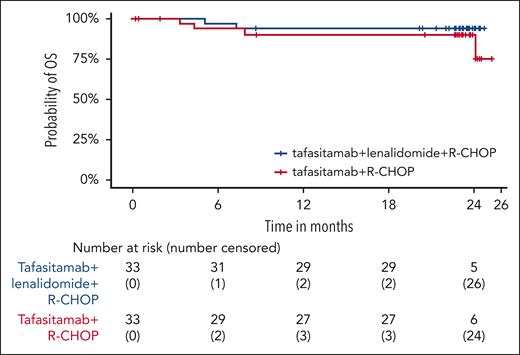
At the final analysis (≥18 months’ follow-up after the EoT visit for all patients), the 18-month DoR and DoCR rates in arm T were 72.7% and 74.5%, respectively, and in arm T/L were 78.7% and 86.5%, respectively (Table 2). Furthermore, at the 24-months’ follow-up, PFS rate was 72.7% in arm T and 76.8% in arm T/L (Figure 4). Similarly, 24-month OS rate was 90.3% in arm T and 93.8% in arm T/L (Figure 5).
Exploratory analyses
Median NK-cell counts decreased at baseline during cycle 1, day 8 but had returned to baseline or higher levels by EoT in arm T and by cycle 1, day 15 in arm T/L and sustained thereafter. T-cell counts decreased at baseline during cycle 1, day 8 in both arms but were at baseline level or higher by cycle 1, day 15 in arm T and by EoT visit in arm T/L and sustained thereafter. Median B-cell counts decreased from baseline to 0 cells/μL in arm T by cycle 1, day 15 and by cycle 1, day 8 in arm T/L. At 6-months’ follow-up after EoT visit, B-cell counts had recovered to measurable levels in ∼50% of patients, indicating a gradual recovery of the B-cell compartment for these patients.
Post hoc analysis
A total of 60.6% of patients in arm T and 66.7% in arm T/L had an IPI score of 3 to 5. In patients with an IPI score of 3 to 5 in arm T/L (n = 22), ORR, 18-month DoR, and DoCR, 24-month PFS and OS rates were comparable with the overall treatment cohort of arm T/L (Table 2). Safety was also comparable with that of the overall arm T/L cohort (Figure 3) (this analysis was not conducted in the arm T subgroup).
Discussion
The phase 1b First-MIND study established the feasibility of adding tafasitamab ± lenalidomide to R-CHOP in the first-line setting in patients with IPI from 2 to 5 DLBCL, demonstrating a manageable safety profile. Furthermore, preliminary efficacy suggests a potential clinical benefit for this regimen in patients who received no previous treatment, and biomarker data support potential future options for prognostic testing.
As mentioned previously, First-MIND builds on data with R2-CHOP in first line, and tafasitamab + lenalidomide in second and later lines, which allowed addition of 2 agents to R-CHOP with the expectation of a manageable safety profile. Across the safety run-in and main study phases, no new safety signals were observed for tafasitamab ± lenalidomide with R-CHOP compared with reported safety from these predecessor trials.6,11,20 The majority of TEAEs reported (842/1248 [67%]) were grade 1 or 2 and were reversible. Grade ≥3 hematologic toxicities including neutropenia, thrombocytopenia, and lymphopenia were numerically higher in patients in arm T/L compared with arm T, whereas grade ≥3 nonhematologic toxicities were similar in both treatment arms. The rate of grade ≥3 febrile neutropenia (18.2%) was identical between the arms, and almost all patients (including all those in arm T/L) recovered within 14 days. Although primary neutropenia prophylaxis with G-CSF or pegylated G-CSF was mandatory and prescribed to all patients, the dose and schedule of the selected drug was decided according to local practice and institutional guidelines. Consequently, the type and duration of G-CSF administration were heterogeneous, and the data from this study do not afford any practical advice for number of doses or duration of treatment. The similar occurrence of febrile neutropenia between arms suggests that the addition of lenalidomide was sufficiently managed as to not result in increases in these important AEs.
With the ARDI of R-CHOP maintained in both treatment arms, the combination of tafasitamab ± lenalidomide does not appear to impede the administration of R-CHOP. In a post hoc analysis, the overall safety profile in arm T/L was similar in the subset of patients with IPI from 3 to 5 (the majority of whom [n = 29/42; 69%] had IPI 3), indicating that tolerability is maintained in patients with worse prognosis.
Cross-trial comparisons must always be made with caution, but it may be illustrative to consider reported rates of hematologic and other AEs in studies using similar regimens. The rate of grade ≥3 neutropenia appears higher with tafasitamab + R2-CHOP (arm T/L) in First-MIND compared with R2-CHOP in both ROBUST and ECOG-ACRIN E1412;6,11 specifically, rates of grade ≥3 neutropenia were 85%, 60%, and 60% in the 3 studies, respectively. In contrast, rates of other hematologic toxicities across the studies were similar; febrile neutropenia occurred in 18%, 14%, and 25%, thrombocytopenia in 36%, 17%, and 34%, and anemia in 27%, 22%, and 29% of patients, respectively. Incidence of grade ≥3 hematologic AEs was lower in arm T of First-MIND vs the comparators discussed, except that febrile neutropenia was the same as in arm T/L. First-MIND was conducted during the main COVID-19 pandemic, and some cases of infection were associated with COVID-19.
The recovery of B cells within 6 months of EoT visit indicates that the addition of tafasitamab alone or in combination with lenalidomide to R-CHOP did not result in additional long-term immune suppression vs that already reported with R-CHOP alone.21
Efficacy was a secondary end point of this study; no formal statistical hypothesis testing was established for sample size calculations, and the study was not powered for efficacy. ORR at EoT visit and best response across all visits were numerically higher in arm T/L compared with arm T, and was supported by favorable outcome measures (DoR, DoCR, PFS, and OS rates), including a trend toward more pronounced DoR in arm T/L. Nevertheless, the proportion of CR was numerically higher in arm T than in arm T/L, perhaps reflecting the influence of the small sample size. A high-risk subpopulation with an IPI score of 3 to 5 in arm T/L experienced efficacy and safety comparable with that of the overall arm T/L cohort, although, again, the sample size was limited.
These results suggest that treatment with tafasitamab + lenalidomide in addition to R-CHOP may provide clinically meaningful efficacy with a manageable AE burden to patients with treatment-naïve DLBCL. The First-MIND study answered its primary objective of investigating whether any additional safety signals evolved when tafasitamab, alone or in combination with lenalidomide, was added to R-CHOP in the first-line DLBCL setting. The study was not designed to measure any difference in efficacy between the 2 arms; however, the addition of the T/L combination to R-CHOP is expected (on the basis of the use of tafasitamab + lenalidomide in second line) to provide more benefit to patients with untreated DLBCL than either agent added to R-CHOP alone. Furthermore, efficacy results at 18- and 24-month time points in this study suggest a trend toward improved outcomes in arm T/L. The use of lenalidomide is carefully managed based on patient characteristics and the emergence of potentially related AEs, such as neutropenia, with active dose optimization to ensure optimal benefit for the individual patient. The combination of tafasitamab + lenalidomide + R-CHOP is being investigated for efficacy vs R-CHOP alone in the ongoing phase 3, double-blind, placebo-controlled, randomized frontMIND study in newly diagnosed patients with high-intermediate and high-risk DLBCL (NCT04824092), which has recently completed recruitment, with primary results due in 2025.19
Acknowledgments
The authors thank Wolfram Brugger, Anirban Lahiry, Bettina Brackertz, Neha Shah, Derek Blair, Nira Hadar, Andrea Sporchia, Dana-Adriana Botesteanu, and Pia Klopfer for their valued contribution to the First-MIND study. Dana-Adriana Botesteanu developed methodology, conducted formal analysis, carried out project administration, provided resources, supervised and validated the study, and contributed to a prior draft of this manuscript.
Medical writing assistance was provided by Roopa Gaonkar and Emma Leah of Syneos Health and funded by MorphoSys AG. The First-MIND study was sponsored by MorphoSys AG. The sponsor participated in the study design, data collection, data analysis, data interpretation, and writing of the report.
Authorship
Contribution: J.M.B., D.B., M.T., and G.S.N. conceptualized the study; J.M.B., S.W., and M.D.-H. performed data curation; J.M.B. did formal analysis; MorphoSys AG was involved in funding acquisition; M.W.-L., M.D.-H., and S.W. carried out project administration; M.D.-H. and members of the steering committee supervised the study; S.W. validated the study; all authors participated in data collection and/or interpretation; J.M.B., M.W.-L., S.W., and M.D.-H. wrote the original draft; all authors performed review and editing of the manuscript and had full access to all the data in the study; and D.B. had final responsibility for the decision to submit for publication.
Conflict-of-interest disclosure: D.B. received consultancy fees from Roche, Gilead Sciences, Janssen-Cilag, Takeda, MorphoSys AG, and Debiopharm Group; travel, accommodations, and expenses from Roche, Gilead Sciences, and Takeda; and research funding from Roche, Gilead Sciences, Janssen-Cilag, Takeda, MorphoSys AG, Pharmacyclics, Archigen Biotech, and Dr Reddy’s Laboratories. M.A. received consultancy from Incyte, Gilead, Karyopharm, Bristol Myers Squibb, and Takeda; travel, accommodations, and expenses from Roche, Gilead, Bristol Myers Squibb, Celgene, and AbbVie; and research funding from Johnson & Johnson, Roche, and Takeda. E.P.P. received consultancy fees from Amgen, Bristol Myers Squibb/Celgene, Sanofi, GlaxoSmithKline, and Incyte; travel, accommodations, and expenses from Amgen, Bristol Myers Squibb/Celgene, and AbbVie; and speaker's bureau fees from Amgen, Bristol Myers Squibb/Celgene, AbbVie, Takeda, Sanofi, and AstraZeneca. P.B.S. received consultancy fees from Roche, AbbVie, Gilead, Janssen, AstraZeneca, Takeda, MSD, Bristol Myers Squibb, Incyte, and BeiGene; research funding from Roche, Takeda, and Incyte; and honoraria from Roche, AbbVie, Gilead, Janssen, AstraZeneca, MSD, Bristol Myers Squibb, Incyte, and BeiGene. M.T. received consultancy fees from AbbVie, Amgen, Bristol Myers Squibb, Gilead, Incyte, Janssen, MorphoSys AG, Roche, Takeda, Celgene, and Novartis and travel, accommodations, and expenses from AbbVie, Bristol Myers Squibb, Gilead, Janssen, Roche, and Takeda; received honoraria from AbbVie, Amgen, Bristol Myers Squibb, Incyte, Janssen, MorphoSys AG, Roche, Takeda, Novartis, Portola, and AstraZeneca; is a member in an entity’s board of directors or advisory committees from AbbVie, Bristol Myers Squibb, Incyte, Janssen, MorphoSys AG, Roche, Takeda, Novartis, and Portola; and is employed by the 1st Faculty of Medicine, Charles University, General Hospital in Prague. J.D. received research funding from MorphoSys AG and Regeneron. M.W.-L. is employed at MorphoSys AG. S.W. is employed at MorphoSys AG. A.M. is employed at MorphoSys AG. M.D.-H. was employed at MorphoSys AG (until 31 December 2021). J.M.B. received consultancy fees from MorphoSys AG, Adaptive Biotechnologies, Verastem Oncology, AstraZeneca, Bristol Myers Squibb, Kymera Therapeutics, X4 Pharmaceuticals, AbbVie, SeaGen, Kura Oncology, Roche/Genentech, Epizyme, and BeiGene and received speaker's bureau fees from SeaGen and BeiGene. G.S.N. received consultancy fees from Celgene, MorphoSys AG, Genentech, Selvita, Debiopharm Group, and Kite/Gilead; received research funding from Celgene, NanoString Technologies, and MorphoSys AG; and is a member on an entity’s board of directors or advisory committees at Celgene, MorphoSys AG, Genentech, Selvita, Debiopharm Group, and Kite/Gilead. The remaining authors declare no competing financial interests.
The current affiliation for M.D.-H is Daiichi-Sankyo Europe GmbH, Munich, Germany.
Correspondence: David Belada, 4th Department of Internal Medicine—Hematology, University Hospital and Faculty of Medicine, Šimkova 870, 500 03 Hradec Králové, Czech Republic; e-mail: beladad@lfhk.cuni.cz.
References
Author notes
Data sharing requests by qualified researchers pertaining to the First-MIND study will be considered only for noncommercial use on a case-by-case basis (to be approved by MorphoSys; daniel.moik@morphosys.com), starting 12 months from acceptance of the manuscript and until 36 months thereafter; approval may be subject to a data access agreement.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![Study design. ∗In the lenalidomide arm, venous thromboembolism prophylaxis with either low-molecular weight heparins or aspirin is mandatory (according to institutional guidelines). †Rituximab (375 mg/m2) and CHOP chemotherapy included cyclophosphamide (750 mg/m2 IV), doxorubicin (50 mg/m2 IV), and vincristine (1.4 mg/m2 [maximum dose = 2 mg] IV) on day 1 of every 21-day cycle and prednisone/prednisolone (100 mg/day PO) on days 1 to 5. The day 1 steroid dose being part of CHOP (100 mg prednisone/prednisolone, or equivalent, PO or IV) could be used as a further component of premedication before tafasitamab infusion. L, lenalidomide; NOS, not otherwise specified; PO, orally; R, randomized; T, tafasitamab.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/16/10.1182_blood.2023020637/2/m_blood_bld-2023-020637-gr1.jpeg?Expires=1763521538&Signature=QjnsFbnkCCsObfb4aJAW72hR2Mlrnt6IfwPVS1XuMcNJWRp86alXKYi61WPIU9TgBM8tzSQHdqlMrT5UuB0hgRL9kl1Tzrw9zFJxailI8jEzV6Ri6p1FKvSIhoDHFnZ2ZN9amOHUGo9vxl7C3zI8QjDOsDR-ex4W0YAnIv~M-lhYL-Gn5wZX3U6NbkC22z-PlfMmhCM0NUjIxnyB5bj~NDayv5X-vl46Fn7PNlYjeC2QG~PXJBQZkkcTq~97D022jkEayvNoIVK1AWF-OruOQSWCjbJ3e2858inQPrOzOWPGIq9rPJB9JDONp6K-XtTwvXpJoBwOKzl9vdvHgUGFDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)