Key Points
Pembrolizumab and vorinostat is well tolerated with a high ORR in RR cHL.
Pembrolizumab and vorinostat is active in RR cHL that is refractory to PD-1 blockade with an ORR of 56% in this subset.
Abstract
This phase 1 study evaluated the addition of vorinostat to pembrolizumab in patients with relapsed/refractory (RR) classical Hodgkin lymphoma (cHL), diffuse large B-cell lymphoma, and follicular lymphoma. We report the results in cases of cHL. Adult patients with RR cHL who had received ≥1 prior lines of therapy and were ineligible for transplantation were treated in a dose-escalation cohort with 2 dose levels (DLs) and then on an expansion cohort at the recommended phase 2 dose (RP2D) in 21-day cycles. Vorinostat 100 mg twice a day (DL1) and 200 mg twice a day (DL2) was administered orally from days 1 to 5 and 8 to 12; all patients received pembrolizumab 200 mg IV every 3 weeks. The primary end point was safety and determination of RP2D. In total, 32 patients with cHL were enrolled, including 30 at DL2 (RP2D); 78% had received prior anti–programmed cell death 1 (anti–PD-1) therapy, and 56% were PD-1 refractory. Grade ≥3 adverse events (AEs) included hypertension (9%), neutropenia (9%), hypophosphatemia (9%), thrombocytopenia (6%), and lymphopenia (6%). Immune-related AEs included grade 1 or 2 thyroiditis (13%), grade 1 rash (6%), and grade 3 esophagitis/duodenitis (3%). The overall response rate (ORR) was 72% and complete response (CR) rate was 34%. Patients refractory to prior PD-1 blockade (n = 18) had ORR and CR rates of 56% and 11%, respectively. Pembrolizumab and vorinostat was well tolerated with a high ORR rate in RR cHL including in anti–PD-1–refractory disease. This trial was registered at www.clinicaltrials.gov as #NCT03150329.
Introduction
Although most patients with classical Hodgkin lymphoma (cHL) are cured with frontline therapy, between 10% and 30% of patients will have primary refractory or relapsed disease.1-4 Some of these patients will be cured via salvage therapy followed by autologous hematopoietic cell transplantation (auto-HCT),5 but many will be ineligible for transplantation, have refractory disease precluding auto-HCT, or experience disease relapse after auto-HCT.6 Despite the introduction of targeted immunotherapies, including brentuximab vedotin (BV) and programmed cell death 1 (PD-1) blockade, few of these patients will achieve long-term remission; therefore, more effective therapies for relapsed/refractory (RR) cHL remain an unmet need.
PD-1 blockade is well tolerated and effective as monotherapy in patients with RR cHL. However, although most patients respond, the CR rate is low. This is important because in RR cHL, depth of response to PD-1 blockade is associated with durability of responses. Although the average duration of response to anti–PD-1 monotherapy exceeds 1 year,7,8 most patients eventually develop resistance, as evidenced by a 5-year progression-free survival (PFS) of <20% for both nivolumab and pembrolizumab.9,10 Patients who are refractory to PD-1 blockade, especially those also resistant to BV, have limited treatment options and poor outcomes. Furthermore, with the growing use of PD-1 blockade in earlier lines of therapy, patients earlier in their course of therapy may present with anti–PD-1 refractory cHL.
Histone deacetylase inhibitors (HDACis) have immunomodulatory effects, including enhancing antigen presentation, recruiting T cells into tumors, and promoting T-cell function.11 Preclinical models in melanoma and lung cancer demonstrated enhanced antitumor activity when HDACis were combined with PD-1 blockade. We hypothesized that combining the pan-HDACi vorinostat with pembrolizumab could improve the depth and duration of response (DOR) to anti–PD-1 therapy as well as restore sensitivity to PD-1 blockade in PD-1–refractory cHL. We conducted a phase 1 dose-finding study with a dose-expansion cohort evaluating the safety and efficacy of pembrolizumab plus vorinostat in patients with RR cHL, diffuse large B-cell lymphoma, and follicular lymphoma. Here, we report the results from patients with RR cHL.
Methods
Patients
This was a single-center phase 1 dose-escalation trial with a planned expansion cohort. Patients who were eligible were aged ≥18 years with RR follicular lymphoma, diffuse large B-cell lymphoma, or cHL whose disease relapsed or progressed after ≥1 prior line of therapy and were ineligible for or had refused transplantation. Originally, prior anti–PD-1 exposure was allowed if patients had a prior objective response to PD-1 blockade. The protocol was then amended to allow patients to enroll regardless of response to prior PD-1 blockade. After completion of the planned dose-escalation and dose-expansion cohort, the protocol was again amended to enroll 10 additional patients with RR cHL refractory to prior PD-1/PD-L1 blockade, defined as a best response of stable disease (SD) or progressive disease (PD) to prior anti–PD-1/PD-L1 therapy or a best response of complete response (CR)/partial response (PR) but patient developed PD during active anti–PD-1/PD-L1 therapy. Additional inclusion criteria were Eastern Cooperative Oncology Group performance status from 0 to 1, total bilirubin (Tbili) ≤ 1.5 × the upper limit of normal (ULN) or direct bilirubin ≤ ULN for patients with Tbili levels > 1.5 × ULN, aspartate aminotransferase (AST)/alanine aminotransferase (ALT) levels ≤ 2.5 × ULN, prothrombin time (PT)/international normalized ratio (INR) ≤ 1.5 × ULN, and activated partial thromboplastin time (aPTT) ≤ 1.5 × ULN. Patients with Gilbert disease could have a Tbili level ≤ 3 × ULN, and patients with liver involvement by cHL were eligible if AST/ALT ≤ 5 × ULN. Hematologic parameters included absolute neutrophil count ≥ 1000/μL, platelets ≥ 75 × 103/μL, and hemoglobin ≥ 8 g/dL without the use of an erythropoiesis stimulating agent within 7 days of assessment; patients with bone marrow involvement by lymphoma were excluded from these parameters. Patients with known active HIV, hepatitis B, or hepatitis C infection, immunodeficiency or having received any immunosuppressive therapy, including systemic corticosteroids within 7 days before study treatment, prior allogeneic HCT within 5 years or active graft-versus-host-disease, prior auto-HCT within 60 days, or active autoimmune disease requiring systemic immunosuppression, history of noninfectious pneumonitis requiring steroids or current pneumonitis, or a corrected QT interval (QTc) > 470 ms using the Fridericia formula were ineligible. All patients provided informed consent, and the study was approved by the institutional review board and conducted in accordance with the principles of the Declaration of Helsinki. The clinical trial was registered as #NCT03150329.
Trial design and study treatment
Patients with any eligible histology were treated in a dose-escalation cohort with 2 dose levels (DLs) using a rolling-6 design12 and then an expansion cohort at the recommended phase 2 dose (RP2D). In DL1, vorinostat was administered orally at 100 mg twice daily from days 1 to 5 and 8 to 12 of each cycle, and in DL2, vorinostat was administered at 200 mg twice daily from days 1 to 5 and 8 to 12 of each cycle. The pembrolizumab dose was 200 mg IV on day 1 of each cycle; the cycle length was 21 days. Treatment could continue for a maximum of 2 years. Patients with PD could continue therapy at the discretion of the treating investigator, provided there were no signs or symptoms of PD, no decline in performance status, and no progressive tumor at critical anatomical sites requiring urgent medical intervention.
Multispectral immunofluorescence tumor analysis
Formalin-fixed paraffin-embedded tumor samples were evaluated using quantitative, automated, 7-color multispectral immunofluorescence (mIF) staining with multispectral imaging to examine the density of and spatial relationships between immune cells, immune checkpoint proteins, and tumor cells in the cHL tumor microenvironment (TME). Only samples from patients with available pretreatment tumor biopsies obtained after the most recent prior line of systemic therapy immediately before enrollment to the study were analyzed. Paired tumor samples obtained at the time of progression after pembrolizumab/vorinostat were also studied. Two Opal-TSA platform mIF panels for cHL were used: CD3, CD4, CD8, PD-1, CD30, and DAPI (4′,6-diamidino-2-phenylindole) (panel 1) and CD56, CD163, CD79a, PAX5, PD-L1, CD30, and 4′,6-diamidino-2-phenylindole (panel 2). mIF images were acquired using a multispectral Vectra 3 imaging system and analyzed using QuPath version 0.3.0 bioimage analysis software.13 An overview of the whole-slide image analysis strategy and representative images for each panel are found in supplemental Figures 1 and 2, available on the Blood website. For detailed methods, see supplemental Methods. Statistical testing for mIF TME results was performed as described in figure legends using GraphPad Prism 9.5.
Gene expression profiling
L-1236 and L-428 cHL cell lines were treated with vorinostat (1 μM) or vehicle control media (dimethyl sulfoxide) for 48 hours. RNA was then extracted (TRIzol, Thermo Fisher), 1 μg of RNA was allocated for library preparation, and 50 bp single-end reads were generated on an Illumina HiSeq 4000 platform at the City of Hope genomics core facility. The quality of raw paired-end reads was assessed using FastQC. RNA-sequencing reads were aligned to a human genome reference (version hg38) using alignment tool STAR (version 2.7.9a). Differentially expressed genes between vorinostat- and control-treated cell lines were identified using R DESeq2 package. Significant differentially expressed genes were defined as those that passed filters of false discovery rate–adjusted P < .05 and fold change ≥ 1.5 or ≤ −1.5.
Study assessments and end points
Safety was monitored continuously, with toxicities assessed using the Common Terminology Criteria for Adverse Events version 4.0. Dose-limiting toxicity (DLT) was assessed in the first 2 cycles and formally defined in the supplemental Materials. Positron emission tomography (PET)–computed tomography (CT) scans were performed at baseline and then at every 4 cycles until PD or off-study therapy; a diagnostic quality CT with IV contrast was acceptable if CR was previously confirmed via PET-CT. Responses were assessed by investigators per the 2014 Lugano Classification.14 The primary end points were safety, tolerability, and determination of the maximum tolerated dose (MTD) and RP2D. Secondary end points included the overall response rate (ORR), CR rate, DOR, PFS, and overall survival (OS). For patients who continued the protocol therapy despite PD, DOR and PFS were calculated up to the time of formal PD.
Statistical considerations
The MTD was defined as the highest dose level at which, at most, 1 of 6 participants had DLT; RP2D would be at, or lower, than the MTD. Participants who were evaluable for DLT during dose escalation needed to complete treatment through the DLT period (first 2 cycles), receive the planned pembrolizumab dose, and miss <30% vorinostat doses during DLT period, unless a DLT occurred. DOR, PFS, and OS were estimated based on Kaplan-Meier product limit method with Greenwood variance estimator, with the confidence interval (CI) estimated based on log-log transformation. For DOR and PFS, failures included disease relapse/progression or death from any cause. DOR and PFS were censored at last follow-up or start of nonprotocol therapy, whichever was earlier.
Results
Patients
In total, 32 patients with cHL were enrolled across the dose-escalation and -expansion cohorts, including 2 at DL1 and 30 at DL2 (RP2D). Patient baseline characteristics are shown in Table 1; 22 (69%) were male, 23 (72%) were Caucasian, and 9 (28%) reported Hispanic ethnicity. The median patient age was 35 years (range, 18-79 years), and 24 (75%) had stage III-IV disease. The median number of prior therapies was 3 (range, 2-10), including 30 (94%) who had prior BV, 21 (66%) who were BV refractory, 25 (78%) who had prior PD-1 blockade, and 18 (56%) who were PD-1 refractory.
Treatment disposition and safety
All 32 patients were evaluable for safety and efficacy. The median number of cycles was 9 (range, 2-36). Thirty-one patients discontinued treatment; 1 patient remains on therapy. Reasons for treatment discontinuation were as follows: PD or lack of response (n = 15), HCT (n = 7), patient preference (n = 2), completion of 2 years of therapy (n = 4), adverse events (AEs; n = 2; skin rash and generalized muscle weakness), and loss to follow-up (n = 1). The most common any grade AEs are listed in Table 2, including hypertension (81%), fatigue (69%), nausea (69%), hyponatremia (66%), diarrhea (50%), abdominal pain (44%), thrombocytopenia (44%), anemia (41%), and leukopenia (41%). The most common grade ≥3 AEs (supplemental Table 1) included hypophosphatemia (9%), hypertension (9%), neutropenia (9%), thrombocytopenia (6%), and lymphopenia (6%). Immune-related AEs (irAEs) occurred in 4 patients with grade 1 or 2 thyroiditis, 2 patients with grade 1 rash, and 1 patient with grade 3 esophagitis/duodenitis who was able to resume therapy and complete 2 years of treatment. One patient had vorinostat dose reduction for neutropenia. At DL2, in >90% of therapeutic cycles (390 of 429 of total cycles administered), patients received ≥ 95% of the intended vorinostat dose. Eight patients died, including 2 post-HCT deaths, 5 due to PD, and another due to sepsis 2 months after PD.
Efficacy
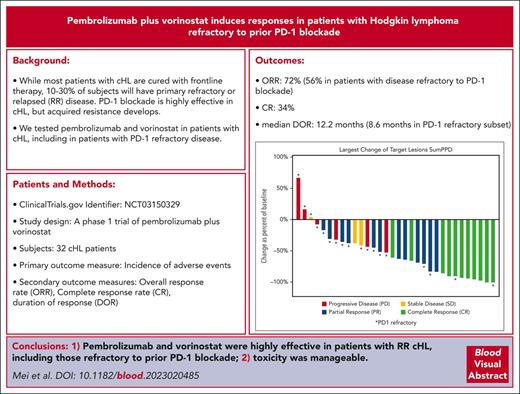
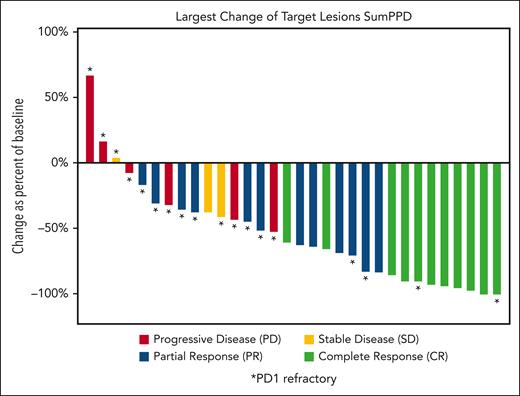
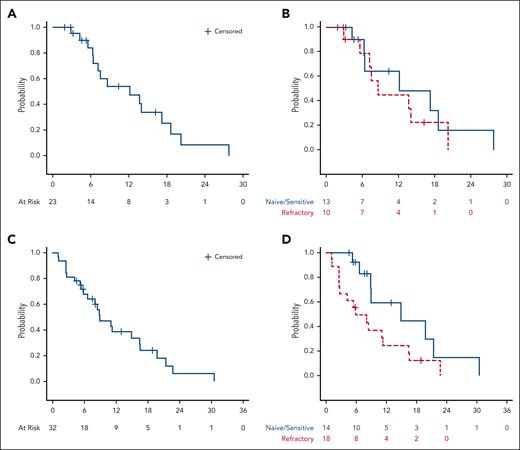
The best ORR was 72%: 11 (34%) patients experienced CR, and 12 (38%) had a PR; 3 (9%) patients had SD, and 6 (19%) patients had PD (Table 3). The percent change in the sum of the product of diameters during treatment is depicted in Figure 1. Among patients who were naive or sensitive to prior anti–PD-1 treatment (n = 14), 13 (93%) patients responded, including 9 (64%) patients with CR. Among patients who were refractory to prior PD-1 blockade (n = 18), 10 (56%) responded, and 2 (11%) had a CR. Twelve patients who were anti-PD1 refractory had an anti-PD1 based regimen as their most recent therapy; 7 (58%) of these patients responded to pembrolizumab/vorinostat, including 1 patient with CR (supplemental Table 2). At data cutoff, the median follow-up time among patients who survived was 32.5 months (range, 16.7-60.3 months). The median DOR among all patients who responded was 12.2 months (95% CI: 6.4-17.2), including a median DOR of 12.2 months (95% CI, 4.3-27.8) and 8.6 months (95% CI, 2.9-20.2) among patients naive/sensitive to anti–PD-1 and those refractory to anti–PD-1, respectively. The 1-year DOR was 54.0% (95% CI, 28.5-73.9) among all patients, 64.3% (95% CI, 24.5-87.1) among patients naive/sensitive to anti–PD-1, and 45.0% (95% CI, 13.9-72.4) among those refractory to anti–PD-1 (Figure 2A-B). The median PFS among all patients was 8.9 months (95% CI, 5.8-16.4); among those naive/sensitive to anti–PD-1 and those refractory to anti–PD-1, the median PFS was 14.9 months (95% CI, 6.6-21.4) and 5.8 months (95% CI, 2.5-11.3), respectively (Figure 2C-D). The 1-year PFS was 38.5% (95 CI, 20.3-56.6) among all patients, and among patients naive/sensitive to anti–PD-1 and those refractory to anti–PD-1, the 1-year PFS was 59.3% (95 CI, 23.3-82.9) and 24.7% (95% CI, 7.8-46.4) respectively. The median OS was not reached, and the 2-year OS was 80.9% (95% CI, 62.2-90.9) among all patients. More information regarding the subset of patients refractory to anti–PD-1 can be found in supplemental Table 3, and retrospective data regarding responses to the next line of therapy after pembrolizumab/vorinostat are provided in supplemental Table 4.
Percentage change in the sum of the product of diameters during treatment in patients with cHL treated with pembrolizumab and vorinostat. PPD, product of the perpendicular diameters.
Percentage change in the sum of the product of diameters during treatment in patients with cHL treated with pembrolizumab and vorinostat. PPD, product of the perpendicular diameters.
Duration of response and progression-free survival in patients with cHL treated with pembrolizumab and vorinostat. DOR in all responders (A), responders stratified as being anti–PD-1 naive/sensitive vs refractory (B); PFS in all patients (C), and patients stratified as being anti–PD-1 naive/sensitive vs refractory (D).
Duration of response and progression-free survival in patients with cHL treated with pembrolizumab and vorinostat. DOR in all responders (A), responders stratified as being anti–PD-1 naive/sensitive vs refractory (B); PFS in all patients (C), and patients stratified as being anti–PD-1 naive/sensitive vs refractory (D).
Seven patients underwent HCT directly after the study therapy (4 autologous and 3 allogeneic). None of the patients who underwent auto-HCT developed engraftment syndrome, and all remain in ongoing response ranging from 14 to 32 months after HCT. Of the 3 patients who received allogeneic HCT, 1 patient was lost to follow-up but was alive without relapse or chronic graft-versus-host disease at 22 months after HCT, 1 patient died of multiply relapsed disease, and the third patient died of invasive fungal infection in the context of steroid-refractory chronic graft-versus-host disease.
TME analysis
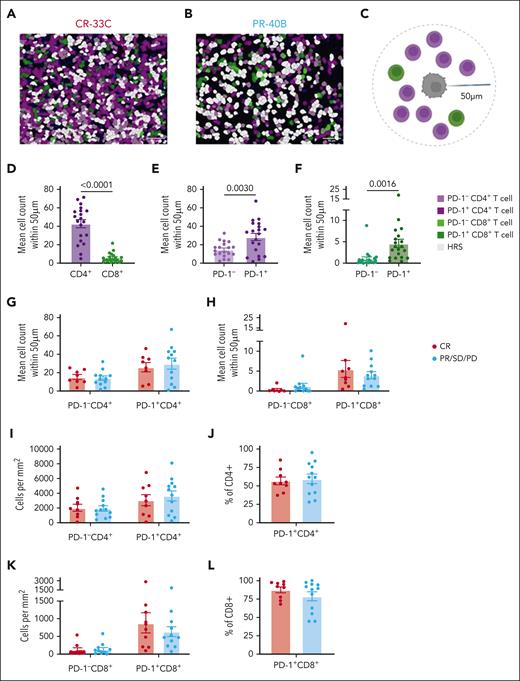
Twenty patients (8 with CR and 12 with non-CR) had available pretreatment tumor tissue (n = 20 evaluable, Panel 1; n = 17 evaluable, Panel 2), and 3 had paired pre- and post-pembrolizumab/vorinostat tumor specimens. In the pretreatment samples, localized densities of CD4+ T cells were higher than that of CD8+ T cells within 50 μm of Hodgkin and Reed/Sternberg (HRS) cells (Figure 3A-D). Among both CD4+ and CD8+ T-cell subsets, PD-1+ T cells were more frequent near HRS cells (Figure 3E-F).Despite this, we found no differences in CD4+, CD8+, or PD-1+ T-cell numbers or frequencies within 50 μm of HRS (Figure 3G-L).
High densities of PD-1+ T cells proximal to HRS cancer cells. mIF imaging was performed to quantify tumor-infiltrating T cells. Cells were phenotyped as PD-1− CD4+ T cells, PD-1+ CD4+ T cells, PD-1− CD8+ T cells, PD-1+ CD8+ T cells, or HRS cells. Representative images (scale bar, 50 μm) with displayed phenotypes are shown for a patient with CR (A) and a patient with PR (B). Cell counts within 50 μm of a HRS cell were quantified (C). Mean counts of total CD4+ and CD8+ T cells (D), PD-1− and PD-1+ CD4+ T cells (E), and PD-1− and PD-1+ CD8+ T cells (F) for each tissue assessed are shown. Mean counts within 50 μm of an HRS cell of PD-1+/− CD4+ T cells (G) and PD-1+/− CD8+ T cells (H) were also compared between patients with CR or PR/SD/PD. Similarly, CD4+ densities (I), percentage of CD4+ T cells expressing PD-1 (J), CD8+ densities (K), and percentage of CD8+ T cells expressing PD-1 (L) in patients with CR compared with those with PR/SD/PD. Statistics are generated using unpaired Student t tests.
High densities of PD-1+ T cells proximal to HRS cancer cells. mIF imaging was performed to quantify tumor-infiltrating T cells. Cells were phenotyped as PD-1− CD4+ T cells, PD-1+ CD4+ T cells, PD-1− CD8+ T cells, PD-1+ CD8+ T cells, or HRS cells. Representative images (scale bar, 50 μm) with displayed phenotypes are shown for a patient with CR (A) and a patient with PR (B). Cell counts within 50 μm of a HRS cell were quantified (C). Mean counts of total CD4+ and CD8+ T cells (D), PD-1− and PD-1+ CD4+ T cells (E), and PD-1− and PD-1+ CD8+ T cells (F) for each tissue assessed are shown. Mean counts within 50 μm of an HRS cell of PD-1+/− CD4+ T cells (G) and PD-1+/− CD8+ T cells (H) were also compared between patients with CR or PR/SD/PD. Similarly, CD4+ densities (I), percentage of CD4+ T cells expressing PD-1 (J), CD8+ densities (K), and percentage of CD8+ T cells expressing PD-1 (L) in patients with CR compared with those with PR/SD/PD. Statistics are generated using unpaired Student t tests.
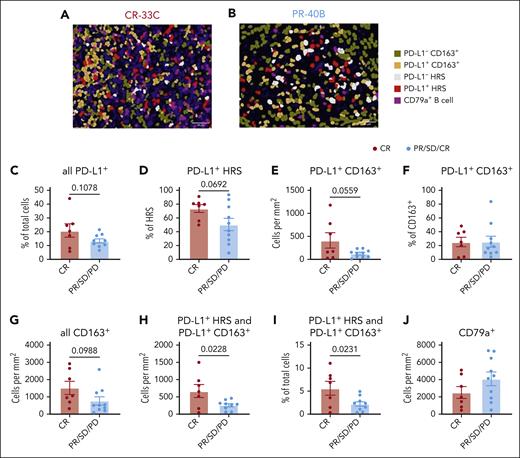
Next, we evaluated the frequency of PD-L1+ cells and CD163+ tumor infiltrating macrophages in the TME (Figure 4A-B) and found that the frequency of PD-L1+ cells among all cells in the TME (P = .1078; Figure 4C), the frequency of PD-L1+ cells among HRS cells (P = .0692; Figure 4D), and the density of PD-L1+ CD163+ cells (P = .0559; Figure 4E) all trended toward association with CR, although the results were not significant. The higher densities of PD-L1+ CD163+ cells did not reflect an increase in frequency of PD-L1+ cells among CD163+ cells but rather a trend toward increased densities of CD163+ cells (P = .0988; Figure 4F-G). We then found that a combined metric of PD-L1+ HRS and PD-L1+ CD163+ density or their combined fraction of total TME cells was significantly associated with CR (P =.0228 and P = .0231; respectively; Figure 4H-I). Finally, we observed no significant association between response and densities of CD79a+ B cells and plasma cells (Figure 4J).
Higher pretreatment fractions of PD-L1+ HRS and PD-L1+ macrophages within the TME are associated with response. mIF imaging was performed to quantify PD-L1 expression, TAMs, and B cells. Cells were phenotyped as PD-L1− CD163+ macrophages, PD-L1+ CD163+ macrophages, PD-L1− HRS cells, PD-L1+ HRS cells, or CD79a+ B cells. Representative images (scale bar, 50 μm) with displayed phenotypes are shown for a patient with CR (A) and a patient with PR (B). The percentage of all TME cells expressing PD-L1 (C), the percentage of HRS cells expressing PD-L1 (D), the density of PD-L1+ CD163+ macrophages (E), the percentage of CD163+ cells expressing PD-L1 (F), and density of all CD163+ macrophages (G) were compared between patients with CR or PR/SD/PD. The combined density of PD-L1+ HRS cells and PD-L1+ CD163+ macrophages (H) as well as their frequency among all TME cells (I) is shown. Similarly, CD79+ B cells densities were compared (J). Statistics are generated using unpaired Student t tests.
Higher pretreatment fractions of PD-L1+ HRS and PD-L1+ macrophages within the TME are associated with response. mIF imaging was performed to quantify PD-L1 expression, TAMs, and B cells. Cells were phenotyped as PD-L1− CD163+ macrophages, PD-L1+ CD163+ macrophages, PD-L1− HRS cells, PD-L1+ HRS cells, or CD79a+ B cells. Representative images (scale bar, 50 μm) with displayed phenotypes are shown for a patient with CR (A) and a patient with PR (B). The percentage of all TME cells expressing PD-L1 (C), the percentage of HRS cells expressing PD-L1 (D), the density of PD-L1+ CD163+ macrophages (E), the percentage of CD163+ cells expressing PD-L1 (F), and density of all CD163+ macrophages (G) were compared between patients with CR or PR/SD/PD. The combined density of PD-L1+ HRS cells and PD-L1+ CD163+ macrophages (H) as well as their frequency among all TME cells (I) is shown. Similarly, CD79+ B cells densities were compared (J). Statistics are generated using unpaired Student t tests.
We examined the number of HRS cells within 50 μm of CD163+ cells and found that PD-L1+ CD163+ cells tended to have more HRS cells nearby (P = .0555) than PD-L1− CD163+ macrophages (supplemental Figure 3A,B); however, PD-L1+ and PD-L1− HRS cells had similar numbers of CD163+ cells nearby (supplemental Figure 3C-D). In patients with CR, both PD-L1+ and PD-L1− HRS cells trended toward having more CD163+ cells nearby (P = .0513 and P = .0774; respectively), with no particular enrichment in PD-L1+ or PD-L1− CD163+ macrophages (supplemental Figure 3E,F). However, from the perspective of CD163+ macrophages, both PD-L1+ CD163+ and PD-L1− CD163+ cells had significantly more PD-L1+ HRS cells nearby in patients with CR as compared with those with non-CR (P = .0270 and P = .0213; respectively; supplemental Figure 3G-J).
We observed no significant differences in densities or PD-1+ frequencies of CD8+ or CD4+ T cells (supplemental Figure 4A-D) or in the densities or PD-L1+ frequencies of HRS cells (supplemental Figure 4E-F) in patients recently treated with PD-1 blockade compared with in patients who were anti–PD-1 naive (n = 14 evaluable, panel 1; n = 12 evaluable, panel 2). Patients with recent PD-1 blockade had significantly decreased densities of CD163+ macrophages (P = .0337; supplemental Figure 4G), and PD-L1− CD163+ macrophages, specifically, were significantly less dense in the TME of patients with recent anti–PD-1 exposure (P = .0221; supplemental Figure 4H-J). Although combined PD-L1+ HRS/CD163+ cells and frequency of HRS cells near CD163+ subsets trended lower in patients with recent anti–PD-1 exposure, no significant differences were identified (supplemental Figure 4K-M). In contrast, the TME of patients with recent anti–PD-1 exposure had higher densities of CD79a+ B cells (P = .0116) and increased numbers of B cells nearby both PD-L1+ and PD-L1− HRS cells (P = .0036 and P = .0025; respectively; supplemental Figure 4N-P).
In 3 patients with paired pretreatment and postprogression biopsy specimen, no significant differences in densities of CD79a+ B cells, CD163+ macrophages, CD8+ T cells, or CD4+ T cells were found (supplemental Figure 5A-D). However, densities of CD8+ and CD4+ T cells increased in 2 of 3 patients. There was increased frequency of HRS+ cells expressing PD-L1 at the time of relapse (P = .0100), which was not observed in CD163+ macrophages (supplemental Figure 5E-F). No differences in fractions of CD8+ or CD4+ T cells expressing PD-1 was found (supplemental Figure 5G-H).
To understand how vorinostat might have increased CD3+ T-cell infiltration and modulated HRS cells to promote response to pembrolizumab, we performed RNA sequencing on vorinostat-treated cHL cell lines. We observed broad transcriptomic changes upon vorinostat treatment, with significant upregulation of genes critical to cytotoxic T-cell recruitment (CXCL9, CXCL10, and CXCL11), T-cell costimulation (CD70, CD80, CD83, CD86, TNFSF4, and TNFSF9), and antigen presentation (B2M, HLA-B, and HLA-DRB). We also observed significant upregulation of CD274 (PD-L1) and downregulation of CCL17, the latter being a critical chemokine that attracts immune-suppressive regulatory T cells (supplemental Figure 6).
Discussion
In our study of pembrolizumab and vorinostat in patients with RR cHL, we observed excellent antitumor activity in a heavily treated cohort of patients with RR cHL. Specifically, we found high OS and CR rates in patients with naive/sensitive anti–PD-1 RR cHL and observed responses in a majority of patients with anti–PD-1 refractory cHL. Responses were durable, with a median DOR of >12 months. The addition of vorinostat to pembrolizumab was well tolerated, with a safety profile consistent with the toxicities of each drug.
The introduction of PD-1 blockade has been an important advancement in the treatment of cHL. However, because most patients with RR cHL treated with anti–PD-1 monotherapy will ultimately relapse, there is room to improve on the current paradigm. Furthermore, as PD-1 blockade is used in earlier lines of therapy increasingly, those patients who do develop RR cHL often have anti–PD-1 refractory cHL. In solid tumors, preclinical studies have identified multiple epigenetic mechanisms contributing to PD-1 resistance,15 as well as other mechanisms of resistance, including influx of myeloid-derived suppressor cells16 and defects in interferon-receptor signaling,17 among others. Immune modulation through HDACi has been explored as a potential avenue to counteract anti–PD-1 resistance. HDACis upregulate PD-L1 expression in cell lines and mouse models across multiple solid tumors as well as in B-cell lymphomas,18-20 and it also suppresses tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells,21 and can stimulate interferon-gamma responses. Multiple clinical trials have evaluated HDACis combined with PD-1 blockade in solid tumors.22 In RR cHL, epigenetic modifying therapy with the hypomethylating agent decitabine was combined safely with the anti–PD-1 antibody camrelizumab and led to a high response rate among patients who were naive or resistant to anti–PD-1.23
We found the toxicities of pembrolizumab/vorinostat to be consistent with the known safety profile of each agent. HDACis, especially vorinostat, can be associated with gastrointestinal toxicity, cytopenias, and fatigue when given continuously. Aiming merely for immunologic augmentation of pembrolizumab while seeking to avoid excess toxicity, we used an intermittent dosing scheme for vorinostat that our group used in a prior study.24 We observed typical vorinostat toxicities, but nearly all were grade 1, and no patients discontinued therapy because of toxicity. Two patients withdrew voluntarily from the study, possibly because of the side effects of vorinostat. We observed a high proportion of hypertension with this combination, suggesting that patients should have regular blood pressure monitoring, and patients receiving antihypertensives may need dose adjustment while on therapy. Importantly, although vorinostat potentially augmented the antitumor activity of pembrolizumab, there was no discernible increase in irAEs, relative to that with pembrolizumab monotherapy.
In our study, pembrolizumab/vorinostat was clinically active and elicited responses in a majority of the patients refractory to PD-1. The CR rate (64%) was high in patients who were naive/sensitive to anti–PD-1, but the cohort was too small to draw conclusions about the additive benefit of vorinostat compared with pembrolizumab monotherapy. The high ORR in patients refractory to prior PD-1 blockade is notable, and most patients (12 of 18) had progressive disease while receiving an anti–PD-1–containing regimen as their most recent therapy. Our findings require validation but do support the notion that the HDAC inhibition played a key role in the antitumor response and reversal of anti–PD-1 resistance, especially because vorinostat monotherapy in cHL has only modest activity, suggesting true synergy with pembrolizumab.25 This is an important finding because patients who have progressed after prior BV and PD1 blockade have limited treatment options, and reversal of acquired PD-1 resistance would represent an important advancement in the treatment of these patients.
In our correlative analyses of tumor specimens, we observed features of the cHL TME that offer possible insights into anti–PD-1 resistance and ways by which HDACis may help overcome it. We noted an increase in the combined density and near proximity of both PD-L1+ HRS cells and PD-L1+ CD163+ TAMs in the TME in patients achieving CR to pembrolizumab/vorinostat. The exact role of TAMs in cHL remains unclear, with conflicting reports regarding the correlation between the number of TAMs in the TME and disease outcomes, potentially because of different effects of M1 and M2 TAM in the TME.26,27 CD163 is a putative marker of immunosuppressive M2 macrophages and there is evidence that HDACis can shift macrophage polarity from a M2 to a M1 phenotype,21 which may account for the deeper responses in patients with more CD163+ macrophages. Moreover, higher densities of CD163+ macrophages and increased PD-L1+ expression among both HRS cells and CD163+ macrophages may reflect a preexisting antitumor T-cell milieu that is further enhanced by pembrolizumab/vorinostat.28
In patients who had paired pretreatment and postprogression biopsies, we observed more PDL1 expression among HRS cells at the time of progression. More T cells were observed in 2 patients at the time of progression, which may be due to PD-1 blockade and HDACi, but further studies are needed to determine how these drugs affect the TME.29,30 Increased PDL1 expression on tumor cells, including HRS cells after HDACi treatment has been observed in preclinical studies,31 and preserved or increased PDL1 expression in patients with cHL who have progressed on anti–PD-1 therapy has been reported in several studies.8,32,33 Our cell line data also support the notion that vorinostat may upregulate CD274 (PD-L1) expression in cHL.34 Moreover, vorinostat significantly increased the expression of genes critical to cytotoxic T-cell traffic, T-cell costimulation, and antigen presentation in HRS cells while significantly decreasing the expression of chemokines important for regulatory T-cell recruitment. Vorinostat may, therefore, directly modulate the gene expression profile of HRS cells in a manner that promotes cytotoxic T-cell infiltration and activation, which could augment antitumor immune responses generated in the context of PD-1 blockade.
Finally, we observed that patients with recent exposure to PD-1 blockade had more B cells and fewer CD163+ TAMs in the cHL TME. Changes in the TME at relapse in patients with cHL resistant to chemotherapy have been described previously, with an observed inverse relationship between B cells and macrophages.35 Increased B cells in the cHL TME in patients with PD after anti–PD-1 therapy has also been described in the literature.36 Because all patients in this study were resistant to prior chemotherapy, this suggests that B cells may be affected by and possibly play a role in resistance to anti–PD-1 therapy. Given that we studied a relatively small cohort of patients with cHL, these observations are merely hypotheses-generating and require validation.
There were limitations to our study. This was a single-center trial, with a small sample size and heterogeneous patient population. Despite this heterogeneity, the majority of patients had heavily pretreated, anti–PD-1 refractory cHL. In another study evaluating epigenetic modification combined with PD-1 blockade, decitabine was administered in a priming prephase before PD-1 blockade in each cycle.23 We reduced logistical complexity by starting pembrolizmab and vorinostat simultaneously, but it is possible that priming with epigenetic modifying agents promotes immunomodulation of the TME before dosing of immunotherapy and affects efficacy. Although most patients who were anti–PD-1 refractory had received PD-1 blockade as their most recent therapy, some received intervening therapy not based on anti–PD-1, and it is possible that retreatment after a break from anti–PD-1 therapy could reinduce an objective response. However, such a response would be expected to be short lived and seems inconsistent with the DOR we observed in these patients. It is possible that pembrolizumab is different enough from nivolumab that a patient who is refractory to nivolumab could simply respond to switching the anti–PD-1 antibody to pembrolizumab, and this has been described in other cancers.37 However, 3 of 4 patients with PD-1 refractory disease who had received pembrolizumab as the most recent therapy before trial enrollment had an objective response to pembrolizumab/vorinostat. Finally, our cell line work was limited by the fact that cHL cell lines do not recapitulate the biologic complexity underpinning the disease given the lack of an accompanying microenvironment; however, there is significant overlap between gene expression seen in microdissected HRS cells and cHL cell lines.38 We acknowledge the inherent limitation but feel the model can serve to generate hypotheses about the clinical effects observed in our study.
In summary, the combination of pembrolizumab and vorinostat was tolerable and effective in a heavily treated cohort of patients with RR cHL. Most patients who were refractory to PD-1 blockade had objective responses, including patients who had PD with PD-1 blockade as their most recent therapy. Our results suggest that the addition of vorinostat to pembrolizumab may enhance and restore sensitivity to PD-1 blockade in patients with RR cHL, supporting the broader concept that epigenetic modifying therapy can sensitize cancers to immunotherapy, meriting further study.
Acknowledgments
Pembrolizumab was provided by Merck Sharp & Dohme LLC. Graphical illustrations were created with Biorender.com.
This work was supported, in part, by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme LLC. A.F.H. was supported by the Emmet and Toni Stephenson Leukemia and Lymphoma Society Scholar Award and the Lymphoma Research Foundation Larry and Denise Mason Clinical Investigator Career Development Award. Research reported in this article included work performed in the Biostatistics and Mathematical Oncology Core and Integrated Genomics Core Facility, supported by the National Cancer Institute, National Institutes of Health under award number P30CA033572.
The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme LLC.
Authorship
Contribution: A.F.H., L.C., and S.P. conceptualized and designed the study; A.F.H., L.C., and M.M. analyzed the data; M.M and A.F.H. prepared the first draft of the manuscript; and all authors collected, assembled, and interpreted the data and helped revise the manuscript.
Conflict-of-interest disclosure: A.F.H. reports research funding from BMS, Merck, Genentech, Inc/F. Hoffmann-La Roche Ltd, Gilead Sciences, Seattle Genetics, AstraZeneca, and ADC Therapeutics; and reports consultancy for BMS, Merck, Genentech Inc/F. Hoffmann-La Roche Ltd, Kite Pharma/Gilead, Seattle Genetics, Karyopharm, Takeda, Tubulis, AstraZeneca, Genmab, Regeneron, Pfizer, AbbVie, Allogene Therapeutics, Adicet Bio, and Caribou Biosciences. M.M. reports research funding from BMS, Incyte, and MorphoSys; reports consultancy for Novartis, Seattle Genetics, CTI, Janssen, and EUSA; and serves on the speakers’ bureaus of MorphoSys and Seattle Genetics. The remaining authors declare no competing financial interests.
Correspondence: Alex F. Herrera, Division of Lymphoma, Department of Hematology/Hematopoietic Cell Transplantation, City of Hope Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: aherrera@coh.org.
References
Author notes
Presented in abstract form at the 60th and 62nd annual meetings of the American Society of Hematology in December 2019 and December 2021, Orlando, FL, and Atlanta, GA, respectively.
Deidentified patient–level data are available on request from the corresponding author, Alex F. Herrera (aherrera@coh.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.