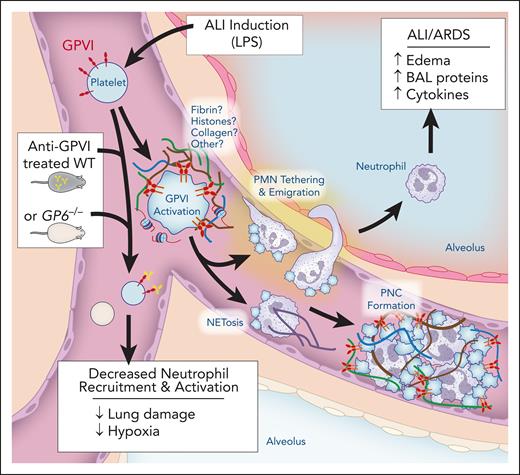
In this issue of Blood, Burkard et al1 demonstrate that platelet glycoprotein VI (GPVI) is a critical regulator of neutrophil recruitment and neutrophil extracellular trap (NET) formation that contributes to pulmonary thromboinflammation in acute lung injury (ALI).
ALI is a significant driver of morbidity and mortality in critically ill patient populations, with >200 000 cases per year in the United States.2 ALI and its more severe counterpart, acute respiratory distress syndrome (ARDS), are characterized by immediate onset of respiratory distress, edema, and hypoxemia.2 This is attributable to breakdown of the lung endothelial barrier, which is driven by the influx of neutrophils generally early in the disease process.2 On infiltration into the lung, neutrophil clustering at sites of inflammation can lead to release of cytokines, reactive oxygen species, and NETs, which further amplify the inflammatory process. Although neutrophil recruitment and activation are well-studied phenomena, the mechanisms by which other cells mediate neutrophil recruitment to the lung during ALI, especially early on, remain unclear. Recent evidence has revealed a significant role for platelets as central figures in mediating inflammatory responses, including neutrophil recruitment.3 However, the platelet signaling events mediating early adhesion events to drive neutrophil recruitment and the mechanism(s) involved in platelet-neutrophil interactions that mediate ALI/ARDS remain unknown. Here, Burkard et al explored the role of platelet GPVI in the early recruitment of neutrophils during ALI using models with either genetic deletion of platelet GPVI or therapeutic inhibition using an anti-GPVI antibody.
Blockade of GPVI reduced neutrophil influx, dampened proinflammatory cytokine levels, and preserved alveolar-capillary barrier function, which are all critical in promoting the development of ALI/ARDS during the initial insult. The inhibition of ALI/ARDS by GPVI blockade indicates platelets play a role in regulating these processes. To directly demonstrate this, the authors visually examined lung tissue to quantify platelet-neutrophil complexes, which were reduced in the absence of GPVI activation. Although these imaging techniques provide fixed snapshots of platelet-neutrophil complexes, intravital imaging additionally confirmed firm neutrophil adhesion in the capillaries was significantly reduced when GPVI was inhibited. These findings suggest that inhibition of these platelet-neutrophil interactions should result in decreased NET formation, which is involved with propagation of lung injury (see figure).
In ALI, inflammation from lipopolysaccharide (LPS) or other pathogens induces generation of unknown platelet GPVI activators. Although fibrin is a strong potential agonist, histones, collagen, or other agonists could be generated to activate GPVI. GPVI activation induces platelet-dependent neutrophil (PMN) recruitment and firm adhesion to the endothelium. This allows for neutrophils to migrate into the alveolar space. In addition, platelet-dependent neutrophil activation can lead to neutrophil extracellular trap formation (NETosis). Overall, the formation of platelet-neutrophil complexes (PNCs) leads to increased edema, brochoalevolar lavage (BAL) proteins, and cytokine generation. Inhibition of GPVI through either genetic deletion or anti-GPVI antibodies decreases neutrophil recruitment and activation, leading to reduced lung damage and hypoxia. Figure prepared with assistance from Diana Lim.
In ALI, inflammation from lipopolysaccharide (LPS) or other pathogens induces generation of unknown platelet GPVI activators. Although fibrin is a strong potential agonist, histones, collagen, or other agonists could be generated to activate GPVI. GPVI activation induces platelet-dependent neutrophil (PMN) recruitment and firm adhesion to the endothelium. This allows for neutrophils to migrate into the alveolar space. In addition, platelet-dependent neutrophil activation can lead to neutrophil extracellular trap formation (NETosis). Overall, the formation of platelet-neutrophil complexes (PNCs) leads to increased edema, brochoalevolar lavage (BAL) proteins, and cytokine generation. Inhibition of GPVI through either genetic deletion or anti-GPVI antibodies decreases neutrophil recruitment and activation, leading to reduced lung damage and hypoxia. Figure prepared with assistance from Diana Lim.
Previously, it was shown that platelets are recruited to the lung before neutrophils, in a process independent of P-selectin or P-selectin glycoprotein ligand-1 (PSGL-1).4 The current study described a critical role for GPVI in mediating platelet recruitment to the lungs and subsequent platelet-neutrophil interactions. However, mechanistically, many unknowns remain about ALI/ARDS-induced platelet activation/recruitment via GPVI. In the lipopolysaccharide-induced ALI/ARDS model described by Burkard et al, inhibition of thrombin and the subsequent prevention of fibrin formation reduced neutrophil recruitment and NETosis. Fibrin is known to regulate platelet activation in a GPVI-dependent manner.5 However, other GPVI agonists are also generated during ALI, including exposure of subendothelial collagen and release of histones, which are both also blocked when thrombin is inhibited. Furthermore, blocking thrombin was previously shown not to affect platelet recruitment in this ALI model.4 This suggests a GPVI ligand unaffected by thrombin regulates lung platelet recruitment after ALI. In addition to the unknown GPVI ligand(s) involved in driving ALI/ARDS, the receptors mediating platelet-neutrophil complex formation remain also unclear. Platelet receptors, including P-selectin, CD40 ligand (CD40L), and activated glycoprotein IIb/IIIa are all expressed on GPVI activation, allowing platelets to interact with neutrophils through their respective ligands PSGL-1, CD40, and CD11b/CD18.3 However, if these specific receptor pairs mediate platelet-neutrophil interactions during ALI remains unknown. The authors note reduced levels of neutrophil CD11b when GPVI is inhibited and imply glycoprotein Ibα (GPIbα) as an additional potential platelet receptor capable of mediating these interactions.1 However, GPIbα-CD11b/CD18 interactions can occur independently of GPVI activation. As the nature of aggregate formation is complex, it is likely multiple receptors pairs are involved.
The severity of the insult inducing ALI/ARDS is an important factor that must be considered if anti-GPVI therapies are to be used in the future to improve outcomes in patients with pulmonary thromboinflammation. Previous studies have demonstrated GPVI is essential for vascular integrity and, in particular, the prevention of inflammatory-induced bleeding.6,7 Whether the lack of GPVI induces inflammatory bleeding is dependent on the vascular bed and severity of the insult. This suggests prevention of drivers of GPVI activation, such as fibrin, histones, or collagen, may be more beneficial than directly targeting the receptor.7 Interestingly, a recent report found an acquired GPVI deficiency in patients with sepsis.8 Future larger clinical studies will need to establish whether this is an endogenous protective mechanism and if it tracks with outcomes of patients with sepsis.
Although platelets play a critical role in the early development of ALI/ARDS, several studies have also suggested platelets play significant roles in the resolution of inflammation. Platelet-induced NETosis is known to prevent bacterial dissemination and can improve outcomes in bacterial sepsis.9 Furthermore, platelet activation and release of CD40L are important in the recruitment of T-regulator cells (Tregs) during ALI/ARDS. The release of CD40L induces transcriptional changes in Tregs necessary to synthesize anti-inflammatory mediators to dampen thromboinflammation in the lung.10 In addition, platelet-dependent recruitment of Tregs is important for macrophage reprogramming and polarization to an anti-inflammatory phenotype.10 Both of these processes are critical for the resolution of inflammation, suggesting that inhibition of these processes later in the disease course through GPVI inhibition could exacerbate ALI/ARDS instead of improving outcomes. Indeed, in a model of pneumonia-derived sepsis, it was shown that GPVI aids in controlling local host defense in the lung through the formation of platelet-leukocyte complexes and by stimulating phagocytosis.9 Thus, although the authors of the current article demonstrate a significant benefit to early GPVI inhibition, more work is needed to fully understand the role of platelet activation mediated by GPVI through the natural progression of the disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests.