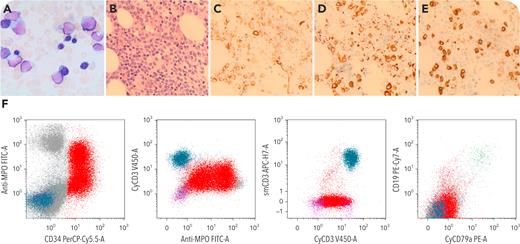
A 72-year-old man presented with anemia and fatigue without hepatosplenomegaly or lymphadenopathy. Complete blood cell count showed severe pancytopenia with few circulating blasts. The bone marrow aspiration was hemodiluted with reduced trilineage hematopoiesis and 30% blasts (panel A; original magnification ×1000; Wright-Giemsa stain; the trephine biopsy revealed hypercellularity with significantly increased blasts (panel B). By immunohistochemistry, CD34+ blasts (panel C; original magnification ×600) were myeloperoxidase (MPO) positive (in a subset) (panel D; original magnification ×600) and CD3+ (in a subset) (panel E; original magnification ×600). Flow cytometry (panel F) demonstrated that the blasts (red) were CD34+/MPO+/CD117+/HLA-DR+/CD13+/CD33+/CD7 (dim), also cytoplasmic CD3 (cCD3+) (intensity reaches that of normal T cells), TdT+, and CD99+; CD19− and cytoplasmic CD79a (cCD79a−). Cytogenetic studies revealed a normal karyotype. Next-generation sequencing detected mutations for biallelic CEBPA (c.992T>C [p.Leu331Pro] allele frequency (AF): 30%; c.68dupC [p.His24Alafs∗84] AF: 21%); CUX1, ASXL1, SETBP1, SRSF2, and TET2. A diagnosis of mixed phenotypic acute leukemia (MPAL) T/myeloid, with mutated CEBPA was rendered.
T/myeloid MPAL is an extremely rare entity generally with poor prognosis. Recent sequencing studies have identified transcription factor CEBPA mutations in a subset of T/myeloid (T/M) MPAL but not in B/myeloid (B/M) MPAL or other types of MPALs, suggesting that CEBPA may play a role in the pathogenesis of the disease. Although biallelic CEBPA mutations defining a specific subtype of acute myeloid leukemia confer a favorable outcome, its clinical impact in T/M MPAL is unclear.
For additional images, visit the ASH Image Bank, a reference and teaching tool that is continually updated with new atlas and case study images. For more information, visit https://imagebank.hematology.org.