In this issue of Blood, Zhang et al1 report new insight into the role of sialyation of glycoprotein (GP) IIb for the detection of maternal alloantibodies (to human platelet antigen [HPA]-9b and HPA-3a). These findings are important for fetal/neonatal alloimmune thrombocytopenia (FNAIT) and extend their previous studies on FNAIT.2 By use of designed induced pluripotent stem cells (iPSCs) differentiated to megakaryocytes (MKs) expressing HPA variants of interest, they elegantly provide data showing maternal alloantibodies in sera can be either sialylation dependent or independent, and that desialylation of glycan chains in close proximity to the allovariant residues has the potential to enhance antibody detection and reveal alloantibodies otherwise missed.
The clinical diagnosis of FNAIT depends on the severity of thrombocytopenia with/without additional explanations for thrombocytopenia and with/without responses to platelet transfusions.3 Serologic/molecular diagnosis requires both fetomaternal (parental) platelet antigen incompatibility and maternal antibodies specific to the incompatible antigen. Antibody detection to HPA-9b has been difficult: here, the authors delineated the role that sialic acid residues can have in detection of anti–HPA-9b, which is novel and important for clinical management.
Why is the diagnosis of FNAIT important? First, thrombocytopenia from FNAIT will resolve. Second, future children of the mother will be at least as severely affected as the current child.4 Thus, maternal treatment should be administered during pregnancy to increase the fetal platelet count. Third, female relatives of the mother may develop alloantibodies to the same platelet antigen. Finally, the consequences of FNAIT are not limited solely to the presence/absence of intracranial hemorrhage (ICH); others, such as reduced birth weight5 and neurodevelopmental abnormalities,6 may also occur.
In White patients, incompatibility of HPA-1a combined with anti–HPA-1a is the dominant cause of FNAIT and is responsible for 70% to 80% of cases.7 Platelet antigen incompatibilities, now numbering >30, may be readily detected by molecular testing, but detection of antibodies is a different story. Although incompatibilities of HPA-3a, HPA-3b, and HPA-9b can result in severe thrombocytopenia and ICH,8,9 detecting their antibodies has been extremely difficult. The same is true for incompatibilities of HPA-15. Thus, if there is severe thrombocytopenia with/without ICH, the assumption of FNAIT is usually made clinically often without serologic confirmation, creating an uncomfortable level of uncertainty regarding the diagnosis. The Versiti Platelet Immunology Laboratory tests 350 to 500 cases of neonatal thrombocytopenia yearly, but only documents FNAIT in one-third of cases (B. Curtis, Versiti, oral communication, 4 October 2023). Are cases being missed by suboptimal testing, or is there sufficient concern to not miss FNAIT that cases unlikely to be FNAIT are being tested? Probably both.
The role of glycosylation—landscaping the protein surface jungle on platelets—has been investigated for decades. Desialylation of platelets leads to platelet clearance in cases of immune thrombocytopenia (ITP).10 However, when it comes to FNAIT, glycosylation of epitopes has slipped under the radar until recently. One reason is that maternal anti–HPA-1a antibodies generally have clear allospecfic reactivity differentiating the 2 variants and are readily detectable. Thus, lack of antibody detection in suspected cases with other antigen incompatibilities has been explained by lack of assay sensitivity.
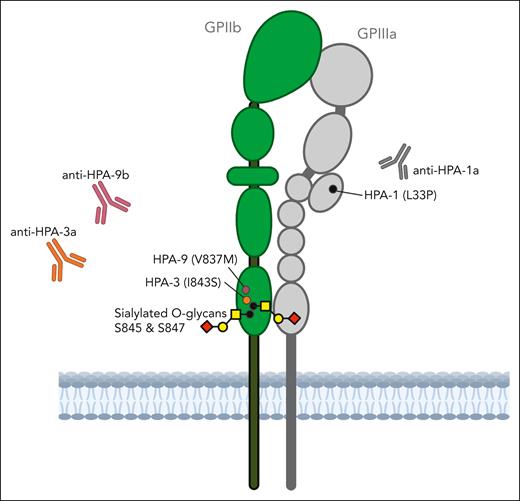
In the current report, HPA-defined, human leukocyte antigen class I–negative MKs differentiated from CRISPR-edited human iPSCs2 are used to explore the effects of desialylation by neuraminidase treatment of glycan chains adjacent to the residues defining HPA-3 and HPA-9 on the reactivity of maternal anti–HPA-3a and anti–HPA-9b alloantibodies. The proximity of the sialylated O-linked glycans at Ser845 and Ser847 to the HPA-3 and HPA-9 is illustrated in the figure. The reactivity of 3 previously characterized anti–HPA-9b sera were enhanced after desialylation, suggesting an unmasking effect of the HPA-9b epitope. Preliminary evidence from 2 cases indicates that maternal anti–HPA-3a antibodies can be either fully sialic acid dependent or independent.
The reactivity of some alloantibodies to HPA-3a and HPA-9b may be sensitive to the sialylation status of the O-linked glycans at nearby serine residues on GPIIb. Figure created with Biorender.com.
The reactivity of some alloantibodies to HPA-3a and HPA-9b may be sensitive to the sialylation status of the O-linked glycans at nearby serine residues on GPIIb. Figure created with Biorender.com.
To further investigate the terminal sialic acid on the glycan vs the effects of the glycan itself on the 2 nearby serine residues of HPA-3 and HPA-9, the authors targeted the serine residues from 2 angles: they bioengineered the HPA-defined iPSC by either genetic knockout of the sialyltransferases involved or Ser-to-Ala substitutions abolishing the glycan site. By this comparison, they elegantly showed that absence of sialic acid enhanced binding, whereas the lack of the glycan chain almost abolished it (although the amino acid substitution could also have had a conformational effect).
Using the designed cells in flow cytometry assays, the authors revealed anti–HPA-9b antibodies in maternal sera in 3 of 8 cases of suspected FNAIT due to HPA-9 incompatibility that were not identified with standard platelet antibody bead array (PABA) testing: 1 of them was detectable with unsialylated cells only.
These findings have implications for the immunization event. The authors speculate that preferential reactivity for epitopes lacking nearby sialylated glycans points to reduced sialylation of antigen at the time of immunization.
Why among many cases of FNAIT with severe neonatal thrombocytopenia only a fraction of cases develop ICH has remained unclear. A leading hypothesis invokes promiscuity of β3 integrin with it being present on vascular endothelial cells and thus potentially being a target of anti–HPA-1a. However, FNAIT cases with antibodies to HPA-9b, HPA-3a, or HPA-3b (all on GPIIB) have also been implicated in FNAIT complicated by ICH, suggesting that the “promiscuity hypothesis” is not the sole explanation for ICH. Also, why these 2 antigens close together on GPIIB (figure) are linked in both having hard-to-detect alloantibodies that may become easier to identify with desiayalation is unclear. Finally, it is not known how many other platelet antigens are similar.
Research is essential for moving FNAIT forward despite limited sample sizes available due to the rarity of the condition. Expanding studies on the use of cell lines, including those with altered glycosylation, may improve antibody testing well beyond the rare cases of HPA-9b incompatibility (HPA-3a and HPA-3b incompatibilities occur in 3%-10% of pregnancies) and may help to delineate the extent to which certain antibodies tend to induce ICH in FNAIT-affected thrombocytopenic neonates. The proof of principle reported here opens up the FNAIT field, and we may anticipate with this and other techniques further sophisticated antigen characterization and improved antibody detection in the future.
Conflict-of-interest disclosure: J.B.B. is a consultant for RallyBio, Janssen, UCB, and Argenx. M.T.A. declares no competing financial interests.