Abstract
Tumor cells in classic Hodgkin lymphoma produce high quantities of the thymus- and activation-related chemokine (TARC). We measured TARC levels in prediagnostic serum samples and found strikingly increased values in the vast majority of patients, as early as 6 years before diagnosis.
TO THE EDITOR:
Classic Hodgkin lymphoma (HL) is a common cancer in young adults, especially in Western world industrialized countries.1 Its tumor cells derive from germinal center B cells and are called Hodgkin and Reed-Sternberg (HRS) cells. In >90% of patients, the HRS cells express thymus- and activation-related chemokine (TARC), also known as CC chemokine ligand 17, which is produced in remarkably high levels. This chemokine attracts CC chemokine receptor type 4–positive CD4 T cells, which may form characteristic T-cell rosettes (ie, clouds of T cells that surround individual HRS cells).2-4 The ensuing interactions between T cells and HRS cells resemble a physiological mechanism in which CD4+ T-helper cells provide essential stimulation to antigen-presenting B cells.5,6 Immunohistochemistry shows that TARC is exclusively expressed by HRS cells, whereas it is not detectable in the abundant and heterogeneous HL tumor microenvironment, nor in the vast majority of other lymphomas.7 The prolific production of TARC by HRS cells is reflected by increased serum levels at the time of diagnosis. These levels are often >100 times higher than in healthy controls and are strongly associated with tumor volume, disease stage, and response to therapy.8-12 Hence, serum TARC (sTARC) levels largely reflect presence and abundance of HRS cells.
In the current study, we hypothesized that sTARC levels are elevated in samples collected before diagnosis. Samples were selected from the US Department of Defense Serum Repository, which harbors a prospective collection of millions of specimens from US active-duty military personnel.13-15 All individuals with an initial diagnosis of HL identified between 1990 and 2000 and with at least 1 prediagnostic serum sample in the 10 years before diagnosis were included. Individuals with a history of HIV infection or malignancy (other than nonmelanoma skin cancer) were excluded. Pathology review confirmed 103 HL cases, of which 23 were positive for tumor cell Epstein-Barr virus (EBV) based on in situ hybridization for EBV-encoded RNAs and/or immunohistochemistry for latent membrane protein-1. Histology subtypes consisted of nodular sclerosis, mixed cellularity, and classic not otherwise specified. For each case, 2 precisely matched controls were selected from the US Department of Defense Serum Repository based on age (±1 year), sex, race and ethnicity, number of serum samples, and sample draw date (±30 days for each sample). The median age was 26 years (range, 18-51 years), and 10% of patients were women (Table 1). TARC levels were measured by a Luminex assay (supplemental Methods [available on the Blood website]). Statistical analyses were performed using linear mixed model analyses that tested the effect of HL case-control status, time to diagnosis, and their interaction on log10-transformed sTARC levels. Case-control pair identifier and individual identifier within a pair were accounted for as random effects. Age and sex were included as potential confounders.
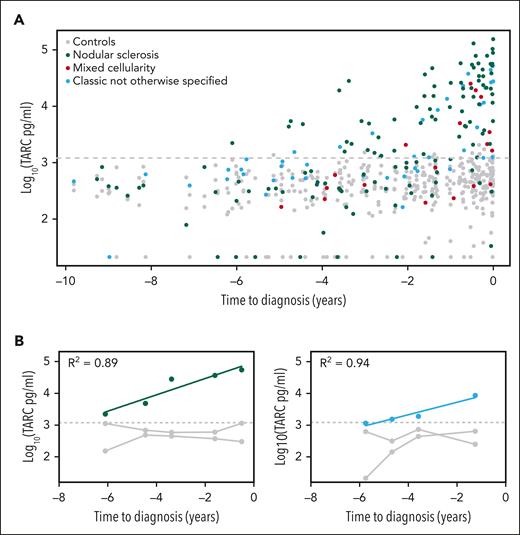
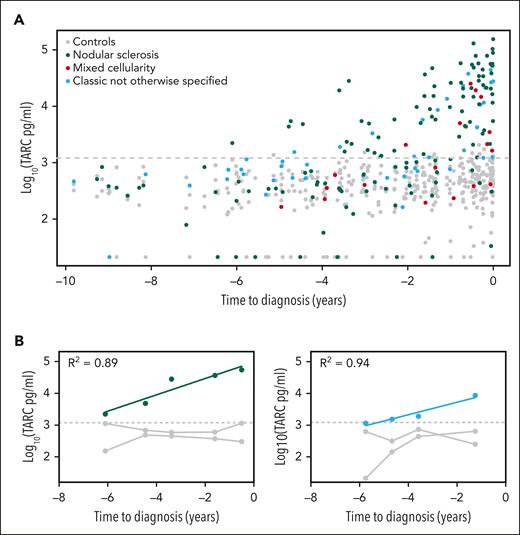
The temporal distribution of sTARC values showed clear differences between cases and controls for both histology subtypes and subgroups defined by tumor cell EBV status (Figure 1A and supplemental Figure 1). Over the entire time span of 10 years before diagnosis, the collective sTARC levels in cases (median, 1305 pg/mL; maximum, 1.5 × 105 pg/mL) were higher than those in controls (median, 407 pg/mL; maximum, 2.1 × 103; P = 2.1 × 10−25). The mixed model analysis on cases and their respective 2 matched controls was even more significant (P = 1.2 × 10−47; Table 2). The corresponding log10 effect size of 1.27 indicates an estimated 18.6-fold higher sTARC level in cases compared with respective controls at the time of diagnosis (95% confidence interval, 13.4-25.9). The largest effect sizes were observed for nodular sclerosis and EBV-negative HL cases (23.3- and 22.5-fold increase, respectively), whereas sTARC levels in the smaller mixed cellularity and EBV-positive subgroups were also clearly increased at diagnosis (estimated 8.2- and 7.6-fold increase, respectively). There were no confounding effects of age and sex. Results of the mixed model analysis per time interval before diagnosis were significant up until 5 years before diagnosis (supplemental Table 1). Log10-transformed sTARC levels increased by 0.20 per year in cases compared with controls, indicating a 1.6-fold increase in sTARC levels every year. This means that sTARC levels start to increase 6.35 years before diagnosis, with some variability in specific HL subsets (Table 2).
Serum TARC levels before diagnosis of classic HL. Log10-transformed serum TARC levels (pg/mL) in the years before diagnosis are plotted for HL cases and their matched healthy controls. The gray dotted line indicates the 95% quantile among the controls (1206 pg/mL). (A) Results of all 103 HL cases and their controls with histology subtype indicated, showing a steady logarithmic increase of serum TARC levels in cases. (B) Results of the 2 cases with multiple positive samples. R2 is the correlation coefficient.
Serum TARC levels before diagnosis of classic HL. Log10-transformed serum TARC levels (pg/mL) in the years before diagnosis are plotted for HL cases and their matched healthy controls. The gray dotted line indicates the 95% quantile among the controls (1206 pg/mL). (A) Results of all 103 HL cases and their controls with histology subtype indicated, showing a steady logarithmic increase of serum TARC levels in cases. (B) Results of the 2 cases with multiple positive samples. R2 is the correlation coefficient.
The young adult age incidence peak is characteristic for HL, but for many of the cases in our study, sTARC levels were already increasing between the ages of 15 and 20 years. TARC is not secreted by normal B cells, leading us to speculate that the events initiating the development of HRS cells occur even earlier, likely during childhood. There is evidence to support that initiating events involve delayed common respiratory tract infections in childhood, which are strongly associated to the young adult HL incidence peak in Western world industrialized countries.16 These respiratory infections often induce activation of regional lymph nodes in the head and neck region or the mediastinum, typical locations of HL manifestation. Other characteristics that link HL to an infectious cause are the germinal center B-cell mutation signature of HRS cells and the association of HL incidence to specific human leukocyte antigen class II types.17,18 We anticipate that as soon as HRS cells acquire production of TARC, they will start to attract CD4-positive T cells, leading to enlargement of the affected lymph node. The importance of TARC and its attraction of CD4+ T cells are corroborated by the observation that HL often shows a pattern of contiguous spread, starting in 1 lymph node and spreading to adjacent ones.19 Local high TARC levels in the lymph node of origin may prepare a CD4+ T-cell rich microenvironment in regional areas, turning these into fertile soil for HRS cells to seed.
Given the long lead time between significantly elevated sTARC levels and clinical recognition of HL, there is a clear potential for screening programs. In this setting, a cutoff value is needed and the probability of false-positive results needs to be considered. Healthy controls in other studies consistently show low sTARC levels (<1000 pg/mL); and in our study, sTARC levels in controls were rarely higher.8,9 In overt atopic dermatitis, sTARC levels can be increased to some extent, and high sTARC levels have also been found in rare malignancies, like primary mediastinal B-cell lymphoma, mycosis fungoides, and Sezary syndrome.7,20-22 Considering the prevalence of atopic dermatitis, there is a possibility of relevant false-positive results in our cohort, but information on atopic disease was not available. We therefore decided to use a 95% quantile cutoff value (ie, 1206 pg/mL). Using this cutoff, 79 of 103 cases had at least 1 positive sTARC test result in their prediagnostic serum samples (supplemental Table 2). For the small subgroup of women, this was 6 of 10. The longest time interval between a positive sTARC test result and HL diagnosis was 6.1 years (Figure 1A).
To evaluate the dynamics of sTARC levels in individual cases, we focused on those with ≥2 consecutive positive sTARC test results (n = 15), including 1 with 5 and 1 with 3 samples. The results of these 2 cases are plotted in Figure 1B and indicate that the increase in TARC levels over time was exponential, which fits well with the log-transformed linear mixed model analysis. The other 13 cases had 2 consecutive positive sTARC test results. For all 15 cases, sTARC levels consistently increased when getting closer to the time of diagnosis. Considering an exponential increase over time, the median lead time to diagnosis was 3.4 years (range, 1.7-7.4 years; supplemental Figure 2).
Although screening for HL using sTARC appears attractive, the incidence of HL in the general population is probably too low to establish a population-based screening program. However, sTARC testing could be considered for individuals presenting with unexplained and painless lymphadenopathy in the head and neck region. The main differential diagnostic consideration in this setting is an exaggerated and self-limiting immune response to some kind of infection. Especially for young individuals, a watch and wait strategy is frequently considered, which may lead to diagnostic delay. How much diagnostic delay could potentially be spared by screening for sTARC remains to be established as remarkably little is known about the prediagnostic dynamics of presenting HL signs and symptoms. Having ≥1 HL affected family members could be an additional trigger for screening, as this increases the lifetime chance of developing HL.23 Because a positive sTARC test result is not entirely specific for HL, it should prompt further clinical investigation, including imaging and tissue biopsy, to ensure accurate diagnosis.
Early detection of HL is highly desirable because it may allow for less intensive treatment and better survival rates. Patients with early-stage HL have an excellent 5-year overall survival and experience fewer treatment-related adverse effects, such as heart disease, infertility, and secondary malignancies, compared with patients with advanced stage HL.24 If screening for sTARC results in a higher proportion of patients being diagnosed at an early stage, this will improve both outcome and quality of life. More important, sTARC levels can be measured by a relatively simple enzyme-linked immunosorbent assay, enabling economical and straightforward testing.9
In conclusion, TARC is strongly involved in HL pathogenesis, and its levels in serum reflect the presence of HRS cells. These levels steadily increase in most patients with HL several years before a clinical diagnosis, presenting novel possibilities for screening strategies with high sensitivity and clinical impact.
Acknowledgments
This work was supported, in part, by the Pendleton Charitable Trust and the McCarthy Family Foundation.
The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70-25.
Authorship
Contribution: A.D., I.M.N., O.M.-M., and L.I.L. designed the study with input from A.v.d.B.; L.I.M. performed the sample measurements under supervision of O.M.-M.; L.I.L. provided quality control on the data; I.M.N. did the statistical analysis; A.D. wrote the first draft of the manuscript; and all authors commented on drafts of the manuscript and approved the final manuscript for submission.
Conflict-of-interest disclosure: A.D. reports research funding from Takeda. The remaining authors declare no competing financial interests.
Correspondence: Arjan Diepstra, Department of Pathology and Medical Biology, University of Groningen, University Medical Center Groningen, Hanzeplein 1, PO Box 30.001, 9700 RB Groningen, The Netherlands; e-mail: a.diepstra@umcg.nl.
References
Author notes
Presented as a selected oral presentation at the 12th International Symposium on Hodgkin Lymphoma, Cologne, Germany, 24 October 2022.
The data sets generated during the current study are available from the corresponding author on reasonable request.
The online version of this article contains a data supplement.