Introduction: Brexu-cel is an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved in the United States for the treatment of adults with R/R MCL and in the European Union for adults with R/R MCL after ≥2 prior therapies, including a Bruton tyrosine kinase inhibitor (BTKi). In the Phase 2 ZUMA-2 study, with a median follow-up of 35.6 months, brexu-cel showed an objective response rate of 91%, a median duration of response of 28.2 months, and a median overall survival of 46.6 months with a manageable safety profile in treated patients (pts) with R/R MCL (N=68). Grade (Gr) ≥3 infections, cytokine release syndrome (CRS), and neurologic events (NEs) occurred in 37%, 15%, and 31% of pts, respectively (Wang et al. J Clin Oncol. 2023). In August 2018, CRS and NE management strategies for ZUMA-2 were updated to initiate treatment of these adverse events earlier at the onset of Gr 1 events to improve safety outcomes. Here, we report 4-yr safety outcomes for pts in ZUMA-2 by initial and updated CRS and NE management strategies.
Methods: Pts with R/R MCL and ≤5 prior treatments, including a BTKi, underwent leukapheresis and conditioning chemotherapy followed by a single infusion of brexu-cel (2×10 6 CAR T cells/kg). For CRS, the initial toxicity management strategy was to consider tocilizumab ± corticosteroids (methylprednisolone 1 mg/kg bid or dexamethasone 10 mg q6h) for Gr 2 CRS with extensive comorbidities or older age and Gr 3 CRS, and to increase the dose of corticosteroids (methylprednisolone 1 g/day x3, followed by a rapid taper consisting of 250 mg bid x2 days, 125 mg bid x2 days and then 60 mg bid x2 days) for Gr 4 CRS. The updated guidance was to administer tocilizumab for Gr 1/2 CRS that does not improve after 24 hours, and tocilizumab + corticosteroids (only if no resolution after 24 hours) and vasopressors for Gr 2 and 3 CRS.
For NEs, the initial guidance was to consider use of tocilizumab for Gr 2 NEs only if other comorbid conditions exist (8 mg/kg IV over 1 hour, not to exceed 800 mg) and to add corticosteroids (dexamethasone 10 mg IV q6h, methylprednisolone 1 mg/kg bid) for Gr ≥3 NEs. In the updated guidance, steroid treatment without concurrent CRS remained the same, but both tocilizumab + dexamethasone 10 mg IV were to be administered for Gr ≥2 NEs concurrent with CRS. Both guidance protocols recommended treatment with antibiotics for management of infections. This exploratory post hoc analysis examined safety outcomes related to CRS, NEs, and infections for pts in each management group, with descriptive statistics reported.
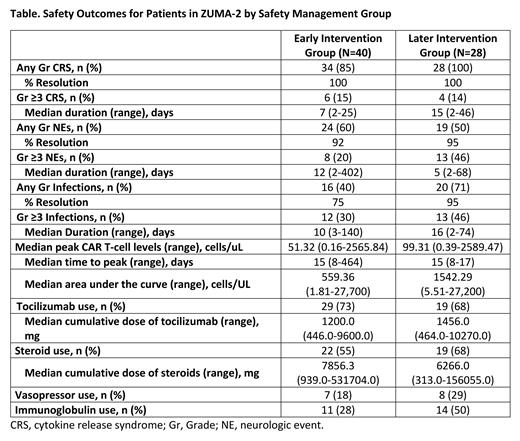
Results: As of July 23 2022, the median follow-up in the 68 treated pts was 47.5 months (range, 37.9-68.3). Of these pts, 40 received updated guidance (early intervention group [EIG]) and 28 received initial guidance (later intervention group [LIG]). Gr ≥3 CRS events were experienced at similar rates in both groups while Gr ≥3 NEs and Gr ≥3 infections occurred less often in the EIG vs the LIG (Table). Gr 5 infections occurred in 2 pts in the EIG and 0 pts in the LIG. No Gr 5 CRS or NEs occurred in either group. The median durations of Gr ≥3 CRS and infections were shorter for the EIG than the LIG while the median duration of Gr ≥3 NEs was longer for the EIG. Any Gr CRS and NEs were resolved at similar rates in both groups, but fewer any Gr infections were resolved for the EIG (all unresolved events in both groups were due to pt death before event resolution). Concomitant medications of interest were used in 80% (n=32) and 82% (n=23) of pts in the EIG and LIG, respectively. Tocilizumab was used at similar rates in the EIG and LIG (73% vs 68%), but steroids (55% vs 68%), vasopressors (18% vs 29%), and immunoglobulins (28% vs 50%) were used at lower rates in the EIG. Median CAR T-cell peak levels and area under the curve were lower for the EIG than the LIG while median CAR T-cell levels peaked at similar times in both groups.
Conclusions: With an updated safety management protocol emphasizing early intervention with tocilizumab and corticosteroids for CRS and NEs, only 20% of pts had Gr ≥3 NEs and 30% had Gr ≥3 infections. Additionally, a lower percentage of EIG pts required steroid, vasopressor, and immunoglobulin use than pts in the LIG. CAR T-cell expansion levels were numerically lower in the EIG. Although limited by small pt numbers, these findings support the current clinical guidance for safety management during brexu-cel treatment, which is close to the ZUMA-2 updated toxicity management guidance that was examined in this study.
Disclosures
Oluwole:AbbVie: Consultancy; ADC: Consultancy, Speakers Bureau; Cargo: Consultancy; Caribou: Consultancy; Epizyme: Consultancy; Kite, a Gilead Company/ Gilead: Consultancy, Research Funding; Nektar: Consultancy; Novartis: Consultancy; TGR: Consultancy; Allogene: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Research Funding; Gilead: Consultancy, Honoraria. Reagan:Seagen: Research Funding; Caribou biosciences: Consultancy; Genentech: Research Funding; Kite, a Gilead Company: Consultancy, Other: speaker. Miklos:Bristol-Myers Squibb: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Adaptive Biotechnologies: Consultancy; Janssen: Consultancy, Honoraria, Other: Travel support; Novartis: Consultancy, Honoraria; Miltenyi: Consultancy, Research Funding; NA: Patents & Royalties: cGVHD patent holder for Ibrutinib as cGVHD therapy but no compensation; Incyte: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; A2 Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria; Juno Therapeutics: Consultancy, Honoraria, Patents & Royalties: rights to royalties from Fred Hutch for patents licensed to Juno, Research Funding; Legend Biotech: Consultancy, Honoraria; Mustang Bio: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Umoja: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Navan Technologies: Consultancy, Current holder of stock options in a privately-held company, Honoraria; 2Seventy Bio: Research Funding; Fate Therapeutics: Research Funding; Allogene: Research Funding; Adicet: Research Funding; Bioline Rx: Membership on an entity's Board of Directors or advisory committees. Locke:Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Leukemia and Lymphoma Society: Other; Aptitude Health: Other: Travel Support; Emerging Therapy Solutions: Consultancy, Other; BioPharma Communications CARE Education: Other: Institutional; Daiichi Sankyo: Consultancy; Society for Immunotherapy of Cancer: Other; Caribou: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional ; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sana: Consultancy, Membership on an entity's Board of Directors or advisory committees; CERo Therapeutics: Other: (Institutional); Cellular Medicine Group: Consultancy; Calibr: Consultancy; National Cancer Institute: Other; Cowen: Consultancy; Imedex: Other; ASH: Other: Travel Support; Wugen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Clinical Care Options Oncology: Other; Legend Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Iovance: Consultancy, Membership on an entity's Board of Directors or advisory committees; EcoR1: Consultancy; GammaDelta Therapeutics: Consultancy; Umoja: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Bristol Myers Squibb/ Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Gerson Lehrman Group (GLG): Consultancy; A2 Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Goy:Hoffman la Roche: Consultancy, Honoraria, Research Funding; Constellation: Research Funding; Alloplex: Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Karyopharm: Research Funding; Infinity: Research Funding; Clinical Advances in Hematology & Oncology: Consultancy; Medscape: Consultancy; Michael J. Hennessey: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Xcenda: Consultancy, Honoraria; Pharmacyclics LLC, an AbbVie Company: Other: Steering Committee, Research Funding; MorphoSys: Research Funding; Practice Update Oncology: Consultancy, Honoraria; COTA Healthcare: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership Role; OMI: Current Employment; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Steering Committee, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genomics Testing Cooperative LLC: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership Role; Acerta: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie/ Pharmacyclics LLC: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Steering Committee, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Other: Steering Committee, Research Funding; Genentech: Research Funding; Vincerx: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other; Verastem: Research Funding; Seagen: Research Funding; Physicians Education Resource, LLC: Consultancy, Honoraria, Other: travel, accommodations, and expenses; OncLive Peer Exchange: Honoraria; Regional Cancer Care Associates, OMI: Current Employment, Research Funding; Resilience: Current holder of stock options in a privately-held company. Jacobson:Morphosys: Consultancy; Instil Bio: Consultancy; Caribou Bio: Consultancy; Abintus Bio: Consultancy; AstraZeneca: Consultancy; ADC Therapeutics: Consultancy; Abbvie: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Novartis: Consultancy; Kite, a Gilead company: Consultancy, Research Funding; Ipsen: Consultancy; Daiichi-Sankyo: Consultancy; ImmPACT Bio: Consultancy; Miltenyi Biotec: Consultancy; Synthekine: Consultancy; Pfizer: Research Funding. Munoz:Genentech/Roche: Consultancy, Research Funding, Speakers Bureau; Epizyme: Consultancy; Beigene: Consultancy, Research Funding, Speakers Bureau; Morphosys/Incyte: Consultancy; MEI: Consultancy; TG Therapeutics: Consultancy; Lilly/Loxo: Consultancy; Incyte: Research Funding; Merck: Research Funding; Portola: Research Funding; Verastem: Consultancy, Speakers Bureau; Celgene: Research Funding; Genmab: Consultancy; Kyowa: Honoraria, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Curio: Honoraria; Alexion: Consultancy; Physicians' Education Resource: Honoraria; Acrotech/Aurobindo: Consultancy, Speakers Bureau; Pharmacyclics/ Janssen: Consultancy, Research Funding, Speakers Bureau; Karyopharm: Consultancy; Celgene/ Bristol-Myers Squibb: Consultancy, Speakers Bureau; Pfizer: Consultancy; ADC Therapeutics: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; OncView: Honoraria; AstraZeneca: Consultancy, Speakers Bureau; Targeted Oncology: Honoraria; Millennium: Research Funding; Bayer: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics/Abbvie: Consultancy, Research Funding. Forcade:Sanofi: Speakers Bureau; GSK: Speakers Bureau; Gilead Sciences: Other: Travel support, Speakers Bureau; Astellas: Speakers Bureau; MSD: Other: Travel support; Alexion: Other: Travel support, Speakers Bureau; Novartis: Consultancy, Other: Travel support, Speakers Bureau. Topp:Regeneron Pharmaceuticals, Inc.: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Other: Travel support, Research Funding; AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; GenMab: Consultancy; Takeda: Research Funding; Janssen: Consultancy, Other: Travel support; Roche: Consultancy, Research Funding. Zheng:Kite, a Gilead Company: Current Employment, Current holder of stock options in a privately-held company, Honoraria, Other: travel support. Nunes:Amgen: Current holder of stock options in a privately-held company; Gilead Sciences Europe Ltd: Current Employment, Current holder of stock options in a privately-held company, Honoraria. Zhang:Kite, a Gilead Company: Current Employment. Shen:Kite, a Gilead Company: Current Employment, Current holder of stock options in a privately-held company, Other: Intellectual property , Patents & Royalties; Atara: Other: Intellectual property , Patents & Royalties. Kloos:Kite, a Gilead Company: Current Employment. Kersten:Kite, a Gilead Company: Consultancy, Honoraria, Other: travel support, Research Funding; Adicet Bio: Consultancy, Honoraria; Takeda: Honoraria, Research Funding; Miltenyi Biotech: Consultancy, Honoraria, Other: travel support; Novartis: Consultancy, Honoraria, Other: travel support; Roche: Consultancy, Honoraria, Other: travel support, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; BeiGene: Other: Travel support; Galapagos: Research Funding. Wang:MD Education: Honoraria; Meeting Minds Experts: Honoraria; Medscape: Honoraria; i3Health: Honoraria; Genmab: Honoraria, Research Funding; Eastern Virginia Medical School: Honoraria; Dava Oncology: Honoraria, Other: Travel; CAHON: Honoraria; Bantam Pharmaceutical: Honoraria; VelosBio: Consultancy, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Parexel: Consultancy; Pepromene Bio: Consultancy; Oncternal: Consultancy, Research Funding; Milken Institute: Consultancy; Miltenyi Biomedicine: Consultancy; Merck: Consultancy, Honoraria; Eli Lilly and Company: Consultancy, Research Funding; Leukemia & Lymphoma Society: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; InnoCare: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Acerta Pharma: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; ADC Therapeutics America: Consultancy; Amphista Therapeutics Limited: Consultancy; Deciphera: Consultancy; DTRM Biopharma (Cayman) Limited: Consultancy; AstraZeneca: Consultancy, Honoraria, Other: Travel, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria; BioInvent: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Be Biopharma: Consultancy; Mumbai Hematology Group: Honoraria; Pharmacyclics: Honoraria; OMI: Honoraria; Nurix: Honoraria; NIH: Honoraria; MJH Life Sciences: Honoraria; Hebei Cancer Prevention Federation: Honoraria; Imedex: Honoraria; TS Oncology: Honoraria; IDEOlogy Health: Honoraria; Oncology Specialty Group: Honoraria; OncLive: Honoraria; Physicians Education Resources (PER): Honoraria, Other: Travel; Practice Point Communications (PPC): Honoraria; Scripps: Honoraria; Studio ER Congressi: Honoraria; WebMD: Honoraria; Practice Point Communications: Honoraria; Physicians Education Resources: Honoraria; CSTone: Consultancy; Celgene: Other: Travel, Research Funding; Genentech: Consultancy, Research Funding; Moffit Cancer Center: Honoraria; Juno Therapeutics: Research Funding; Loxo Oncology: Consultancy, Research Funding; Molecular Templates: Research Funding; Vincerx: Research Funding; Epizyme: Consultancy, Honoraria; Clinical Care Options: Honoraria; BGICS: Honoraria; Anticancer Association: Honoraria.