Patients with TP53-mutant acute myeloid leukemia (AML) constitute the worst outcome group with a survival of only several months. TP53 mutations frequently become dominant following exposure to chemotherapeutic agents, Bcl-2 or MDM2 inhibitors, and confer resistance to multiple other therapies.
Y220C is a recurrent hotspot TP53 mutation observed in solid tumors and hematological malignancies, predominantly in AML and MDS (Gener-Ricoset al., ASH 2022). PC14586 (PMV Pharmaceuticals) is designed to bind to the p53-Y220C mutant and restore wild-type (WT) p53 protein conformation and function. PC14586 has been shown to convert Y220C-p53 mutant into WT-p53 protein conformation, induce the activation of p53 transcriptional pathways resulting in selective inhibition of proliferation ofTP53-Y220C mutant solid tumor cell lines and achieved partial responses and stable disease in an ongoing phase I clinical trial in solid tumors (Dumbrava, ASCO 2022, NCT04585750). However, the activity of PC14586 in TP53-Y220C mutated AML/MDS has not been investigated, either as monotherapy or in mechanism-based drug combinations.
We investigated the mechanisms of action of PC14586 in AML using cells with TP53-WT, knockout (KO), and mutations (one allele with Y220C or R175H and the other truncated) generated by CRISPR (Boettcher et al., Science 2019) and found that PC14586 converts Y220C mutant p53 to the active form of p53 and selectively activates p53 signaling proteins including MDM2 and p21 in TP53-Y220C AML cells. PC14586 decreased cell viability but had limited apoptogenic activities in the TP53-Y220C AML cells . In primary AML cells with Y220C mutations, PC14586 induced cell death in bulk AML cells and, importantly, also in stem/progenitor cells in 2/3 samples.
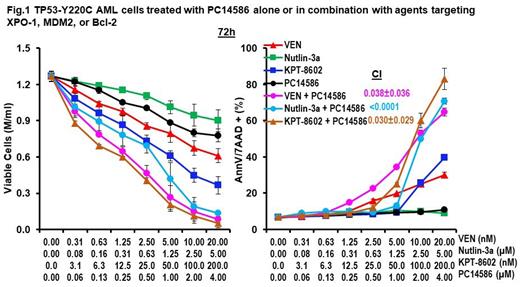
We reported previously that sustained nuclear retention of p53 by XPO-1 inhibition greatly enhances the transcriptional activity of activated p53 and that activation of p53 by inhibiting its negative regulator MDM2 effectively induces cell death in TP53-WT AML (Kojima et al., Blood 2013, 2005), and that XPO-1 inhibition vastly increased apoptosis induced by MDM2 inhibition through 20-60-fold increased p53 transcriptional activities. We also reported that combined Bcl-2 inhibition and p53 activation is synthetically lethal in TP53-WT AML (Pan et al., Cancer Cell 2017). We hypothesized that XPO-1 synergizes with PC14586 by retaining PC14586-activated p53 in the nucleus and that inhibition of MDM2 induced by PC14586-mediated p53 activation or inhibition of Bcl-2 synergizes with PC14586 in TP53-Y220C AML cells. Indeed, we found that PC14586-induced p53 target proteins were further upregulated when PC14586 was combined with XPO-1 or MDM2 inhibition. Inhibition of XPO-1, MDM2, or Bcl-2 synergistically induced massive apoptosis and decreased cell viability in PC14586 treated TP53 Y220C mutant AML cells (Fig.1), in primary AML blasts, and in CD34+CD38- stem/progenitor cells but not in normal bone marrow cells or stem/progenitor cells through conformational changes of mutant p53 to p53-WT. While PC14586 had limited activity on proliferation in TP53-Y220C AML cells, the combination with Bcl-2 inhibition largely blocked G1-S transition and enhanced apoptosis. The G1-S transition block and apoptosis induction were even more pronounced when PC14586 was combined with XPO-1 or MDM2 inhibitors as determined by flow cytometry measurement of DNA content, and PARP cleavage. Experiments in TP53-Y220C AML PDX models are ongoing and will be reported.
In summary, PC14586 converts Y220C-mutant p53 into WT-p53 conformation and induces p53 target proteins. Mechanism-based combinations with XPO-1, MDM2, and Bcl-2 inhibitors induce massive apoptosis. PC14586 is presently in early clinical trials.
Disclosures
Carter:PMV: Research Funding; Revolution Medicines: Research Funding; Syndax: Research Funding; PinotBio: Research Funding. Nishida:Kintor Pharmaceutical: Research Funding. DiNardo:BMS: Honoraria; Novartis: Honoraria; Fogham: Honoraria; Schrödinger: Consultancy; ImmuniOnc: Honoraria; Takeda: Honoraria; AbbVie/Genentech: Honoraria; Astellas: Honoraria; Notable Labs: Honoraria; Servier: Honoraria. Puzio-Kuter:PMV: Current Employment. Levine:PMV: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: co-founder; Chugai Pharma: Consultancy. Andreeff:Kintor Pharmaceutical: Research Funding; PMV: Research Funding.