Introduction: Treatment (Tx) options for T-cell non-Hodgkin lymphomas (T-NHLs) are limited and prognosis is too often poor for patients (pts) with relapsed or refractory (R/R) disease. Enhancer of zeste homolog (EZH)2 and EZH1 are methyltransferases that catalyze trimethylation of histone H3 at lysine 27 (H3K27me3); this repressive transcriptional profile is broadly associated with gene silencing.
Valemetostat tosylate (valemetostat) is a novel, potent, and selective dual inhibitor of EZH2 and EZH1 that prevents H3K27me3 and increases expression of genes involved in immune function ( SLA, PAG1) that can be silenced by H3K27me3. Valemetostat was approved in Japan for the Tx of R/R adult T-cell leukemia/lymphoma (ATLL) in 2022.
The clinical activity of valemetostat in pts with R/R NHLs was investigated in the phase 1 DS3201-A-J101 (“J101”; NCT02732275) trial. Interim data were reported previously. Here we report primary outcomes for pts in J101 with R/R T-NHLs treated with valemetostat.
Methods: This open-label, multicenter study was conducted in Japan and the United States (US). Eligible pts were ≥ 18 (US) or ≥ 20 (Japan) years (y) of age and relapsed from, refractory to, or ineligible for standard therapies. The trial included a dose-escalation phase (Japan only) followed by a dose-expansion phase (Japan & US). Enrollment in the expansion phase was limited to pts with R/R peripheral T-cell lymphoma (PTCL) or ATLL. Pts received valemetostat once daily in continuous 28-day (d) Tx cycles at 150-300 mg/d in the escalation phase and 200 mg/d in the expansion phase until disease progression or intolerance.
Key endpoints for the dose-escalation and dose-expansion cohorts were safety and PK parameters. Preliminary efficacy assessment included objective response rate (ORR), complete response (CR) rate, duration of response (DOR), and progression-free survival (PFS). DOR and PFS were estimated using Kaplan-Meier methods. Results for PTCL and ATLL are reported separately.
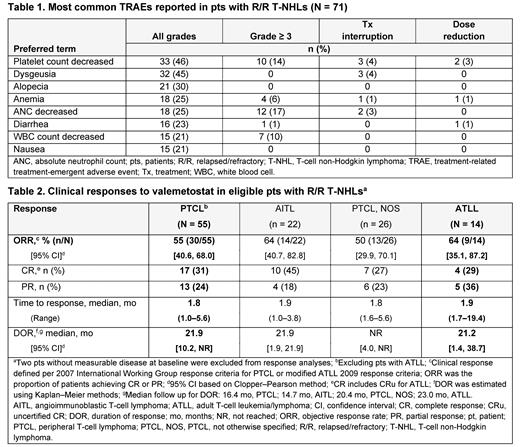
Results: At the primary data cutoff (Dec 31, 2022), 71 pts were included in the efficacy and safety analyses; 57 with PTCL and 14 with ATLL. Median age at baseline was 68.0 (range 26-83) y in the PTCL group and 66.5 (37-78) y in the ATLL group, and median prior therapies were 2.0 (1-8) and 2.5 (1-8), respectively; 16 (28%; 14 autologous, 2 allogeneic) pts with PTCL and 2 (14%, both allogeneic) with ATLL had previously undergone transplant. The most common PTCL subtypes were PTCL, not otherwise specified (PTCL, NOS; n = 26, 46%) and angioimmunoblastic T-cell lymphoma (AITL; n = 23, 40%). Tx was ongoing for 8 (11%) pts at data cutoff.
All 71 pts included at data cutoff experienced ≥ 1 Tx-emergent adverse event; 59 (83%) pts had a Tx-related adverse event (TRAE). The most common all-grade TRAEs were cytopenias, dysgeusia, and alopecia; the most frequent grade ≥ 3 TRAEs were decreased neutrophil (17%), lymphocyte (11%), and platelet (14%) counts ( Table 1). TRAEs led to Tx discontinuation for 4 (6%) pts (1 each: acute myeloid leukemia, myelodysplastic syndrome, colitis, and acute kidney injury), dose reduction for 5 (7%) pts, and Tx interruption for 18 (25%) pts.
The ORR was 55% (30/55) in the PTCL group and 64% (9/14) in the ATLL group, with CR rates of 31% (17/55) and 29% (4/14), respectively; within the PTCL group, the ORR among evaluable pts was 50% (13/26) for PTCL, NOS and 64% (14/22) for AITL ( Table 2). Median times to response were 1.8 (range 1.0-5.6) months (mo) in the PTCL group and 1.9 (1.7-19.4) mo in the ATLL group. Median DOR was 21.9 mo (95% confidence interval [CI], 10.2 mo to not reached [NR]) in the PTCL group (median follow-up 16.4 mo) and 21.2 (95% CI, 1.4 to 38.7) mo in the ATLL group (median follow-up 23.0 mo). Median PFS was 7.7 and 4.1 mo, respectively (median follow-up 18.2 mo and NR).
Conclusions: Valemetostat was well tolerated and showed encouraging clinical activity in pts with R/R T-NHLs. Cytopenias were common but could usually be managed without Tx discontinuation. Valemetostat induced durable responses, with median DOR of > 1.5 y in both the PTCL and ATLL groups. Results for pts in this trial with R/R B-NHLs (Izutsu et al) and from a phase 2 trial in R/R T-NHLs (Horwitz et al) are reported separately.
Disclosures
Jacobsen:Pharmacyclics: Research Funding; Merck: Honoraria, Research Funding; Celgene: Research Funding; Hoffman-LaRoche: Research Funding; Daiichi: Honoraria; BMS: Honoraria; Bayer: Honoraria; UpToDate: Patents & Royalties. Maruyama:Sanofi: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Eisai: Honoraria, Research Funding; Taiho: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; SymBio: Honoraria; Celgene: Honoraria, Research Funding; Zenyaku: Honoraria; Mundipharma: Honoraria; Novartis: Research Funding; AstraZeneca: Honoraria; Ono: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Otsuka: Research Funding; AbbVie: Honoraria, Research Funding; MSD: Honoraria, Research Funding; Nippon Shinyaku: Honoraria; Chugai: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; Astellas: Research Funding; Amgen Astellas Biopharma: Research Funding. Porcu:Kymera: Membership on an entity's Board of Directors or advisory committees; Kyowa: Consultancy; BioGene: Membership on an entity's Board of Directors or advisory committees; Dren-Bio, ADCT, Lilly-Loxo, Viracta, Innate Pharma: Membership on an entity's Board of Directors or advisory committees; Ono: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa, Daiichi, Viracta, Dren Bio, Innate Pharma: Consultancy; Kyowa, Daiichi, Viracta, Dren Bio, Innate Pharma, Ono: Honoraria; Teva: Research Funding; Innate Pharma: Research Funding. Tobinai:Celgene: Honoraria; Daiichi Sankyo: Consultancy, Honoraria; HUYA Bioscience International: Consultancy, Honoraria; Zenyaku Kogyo: Consultancy, Honoraria; Solasia Pharma: Honoraria; Mundipharma: Consultancy, Honoraria. Allen:Kyowa Kirin: Consultancy; Daiichi Sankyo: Consultancy; Secura Bio: Consultancy; Seattle Genetics: Consultancy. Ishitsuka:Takeda: Honoraria; Chugai Pharmaceutical: Honoraria; Bristol Myers Squibb: Honoraria; Abbvie: Honoraria; CSLbehring: Honoraria; Eizai: Honoraria; Genmab: Honoraria; Janssen: Honoraria; Nippon Kayaku: Honoraria; Nippon Shinyaku: Honoraria; Novartis: Honoraria; Ono Pharmaceutical: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; Otsuka: Honoraria; Yakult: Consultancy; Ono Pharmaceutical: Research Funding; Meiji Seika: Consultancy, Honoraria; Kyowa Kirin: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria. Tsukasaki:Meiji Seika Pharma: Consultancy, Honoraria, Research Funding; Daiich-Sankyo: Consultancy, Research Funding; HUYABIO: Consultancy, Research Funding; Ono Pharma: Consultancy; Solasia Pharma: Consultancy; Yakuruto: Consultancy; Kyowa-hakko/Kirin: Research Funding; Bristol Myers Squibb: Research Funding; Bayer: Research Funding; Regeneron Pharmaceuticals Inc.: Research Funding; Chugai Pharma: Honoraria; Eizai: Honoraria; Takeda: Honoraria. Kusumoto:Eisai: Honoraria; Ono: Honoraria; Shionogi: Research Funding; Bristol Myers Squibb: Research Funding; Janssen: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Takeda: Honoraria; Eil Lilly: Honoraria; AstraZeneca: Honoraria; Nippon-Shinyaku: Honoraria; Meiji-Seika: Honoraria; Kyowa-Kirin: Honoraria; Astellas: Honoraria; AbbVie: Honoraria; SymBio: Honoraria; Mundipharma: Honoraria. Foss:Astex: Honoraria; Conjupro: Honoraria; Kyowa: Honoraria; Daiichi Sankyo: Honoraria; SecuraBio: Honoraria; Seagen: Speakers Bureau; Acrotech: Speakers Bureau. Yamauchi:Ono: Research Funding; Takeda: Research Funding; Genmab: Research Funding; Incyte: Research Funding. Morishima:Abbvie: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; Daiichi Sankyo: Honoraria; Kyowa Kirin: Honoraria; Chugai Pharma: Honoraria; Janssen: Honoraria. Imaizumi:Sanofi: Honoraria; Chugai Pharmaceuticals Co., Ltd.: Honoraria; Meiji Seika Pharma Co., Ltd.: Honoraria; Daiichi Sankyo Ltd.: Honoraria; Bristol Myers Squibb: Honoraria; SymBio Pharmaceuticals Ltd.: Honoraria; AstraZeneca: Honoraria. Izutsu:Zenyaku Kogyo: Consultancy; Kyowa Kirin: Honoraria, Research Funding; Chugai Pharma: Honoraria, Research Funding; Regeneron: Research Funding; Janssen: Honoraria; Loxo Oncology: Research Funding; Beigene: Research Funding; Astellas Amgen: Research Funding; Nippon Shinyaku: Consultancy; Mitsubishi Tanabe Pharma: Consultancy; Pfizer: Honoraria, Research Funding; MSD: Honoraria, Research Funding; Nihon Kayaku: Honoraria; Ono Pharmaceuticals: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; Genmab: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Incyte: Research Funding; Novartis: Honoraria, Research Funding; Yakult: Research Funding; Eli Lilly: Honoraria; SymBio Pharmaceuticals: Honoraria; Meiji Seika: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Eisai: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Otsuka: Consultancy, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding. Feldman:ADCT: Consultancy; ADCT: Research Funding; Seagen: Speakers Bureau; ADCT: Honoraria; ADCT: Membership on an entity's Board of Directors or advisory committees; Seagen: Membership on an entity's Board of Directors or advisory committees; KITE: Membership on an entity's Board of Directors or advisory committees; Epizyme: Speakers Bureau; Takeda: Speakers Bureau; Epizyme: Honoraria; Seagen: Honoraria; KITE: Honoraria; BMS: Research Funding; Juno: Research Funding; Janssen: Research Funding; Tessa: Research Funding; Seagen: Research Funding; AstraZeneca: Consultancy; AstraZeneca: Research Funding; Gilead: Consultancy; Seagen: Consultancy; Genmab: Research Funding; Corvus: Research Funding; Kymera: Research Funding; Merck: Research Funding; Takeda: Research Funding; Genomic Testing Cooperative: Current equity holder in private company; BMS: Consultancy; Daiichi Sankyo: Research Funding; Epizyme: Research Funding; Epizyme: Consultancy; Genmab: Honoraria; Genmab: Consultancy; AstraZeneca: Honoraria; Wyeth: Research Funding. Kakurai:Daiichi Sankyo Co., Ltd.: Current Employment. Yamauchi:Daiichi Sankyo Co., Ltd.: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Biserna:BMS: Current equity holder in publicly-traded company; Daiichi Sankyo: Current equity holder in publicly-traded company; Daiichi Sankyo Inc.: Current Employment. Inoue:Daiichi Sankyo Inc.: Current Employment. Tsutsumi:Daiichi Sankyo Inc.: Current Employment. Horwitz:Millenium: Research Funding; Takeda: Consultancy, Research Funding; Affimed: Research Funding; Cimieo Therapeutics: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; Kyowa Hakko Kirin: Consultancy, Research Funding; ONO Pharmaceuticals: Consultancy; SecuraBio: Consultancy; Abcuro Inc.: Consultancy; Auxilius Pharma: Consultancy; Seattle Genetics: Research Funding; Yingli Pharma Limited: Consultancy; Tubulis: Consultancy; Shoreline Biosciences, Inc.: Consultancy; Verastem/SecuraBio: Research Funding; Crispr Therapeutics: Research Funding; Celgene: Research Funding; ADC Therapeutics: Research Funding; Trillium Therapeutics: Consultancy, Research Funding.