Introduction: Genomic profiling in myeloid cancers is vital for disease classification, risk stratification, and treatment decisions. Whole genome sequencing (WGS) captures a complete range of biomarkers, overcoming conventional diagnostic constraints of multiple assays (Duncavage et al, 2021). Duncavage et al's seminal work demonstrated WGS as an alternative and sensitive approach to cytogenetics. Due to emerging evidence, NCCN integrated WGS in their AML testing guidelines (v2.2023). Given the importance of WGS in myeloid cancers, we developed Isabl GxT Heme, an analytical platform, that uses unmatched WGS data as input, assesses all mutation types across the entire genome (structural variants (SVs) and copy number variations (CNVs), small mutations) and delivers an automated report of clinically relevant findings in accordance to latest clinical guidelines. Here we use the Duncavage dataset to analytically validate this WGS approach for the Isabl platform.
Methods:Data for 263 samples from Duncavage et al were processed using Isabl GxT Heme analytical framework. The cohort included 175 AML, 81 myelodysplastic syndrome (MDS), and 7 other myeloid malignancies, with a median coverage of 50x (19x - 90x). 2 samples were excluded due to low coverage (<20x). Sensitivity analysis for Isabl GxT Heme and ChromoSeq (reference test; Duncavage et al., 2021) involved comparing against clinical panel sequencing for single nucleotide variations (SNVs) and indels, and against cytogenetics for SVs and CNVs (restricted to clonal events).
Results:Isabl GxT analytical workflow returned results within one day from FASTQ generation (<19 hours). Isabl GxT Heme and the reference test detected 100% of recurrent SVs (41/41) and clonal CNVs (67/67) that were reported by cytogenetics. Isabl GxT Heme sensitivity for SNVs and indels was 93% (219/236) and 93% (109/117) for all events called by panel sequencing. Sensitivities for the reference test were 85% and 92%, respectively.
A total of 850 calls from 261 samples from the reference test were evaluated next (41 SVs, 205 SNVs, 113 Indels, and 491 CNVs). Isabl GxT Heme identified 100% of SVs, while sensitivity for SNVs was 99.5% (204), and that for indels was 98% (111). Isabl GxT Heme called 97% (475) of CNVs with clonality >20%. Our sensitivity in calling FLT3 internal tandem duplication (ITD) as compared to polymerase-chain-reaction (PCR)-based assay was 84% (27/32) as compared to the reference test at 87% (28/32).
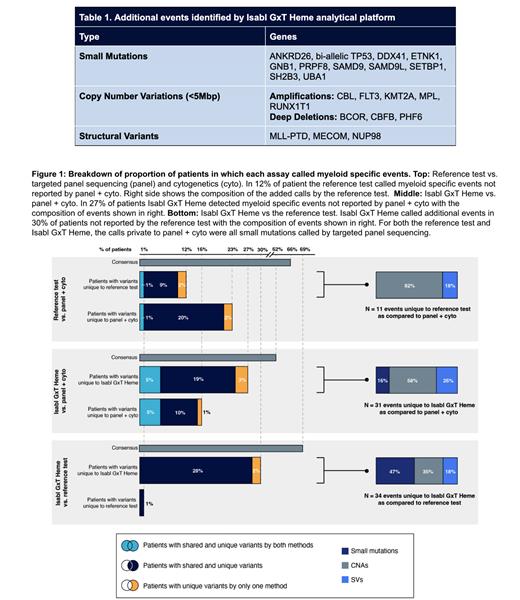
Of the 46 genes from the latest guidelines for myeloid disease classification and risk stratification (guideline genes; WHO-2022, IPSSM-2022), 12 were not reported by the targeted analytical approach of the reference test and panel sequencing. A total of 32 guideline related small mutations in 29 samples (11%) were called by Isabl GxT Heme alone . In 13/29 samples, the called events were deemed oncogenic/likely oncogenic by OncoKB (Chakravarty et al. 2017). Isabl GxT Heme's allele-specific approach detects copy-neutral loss of heterozygosity (cnLOH), identifying 7 samples (3%) with bi-allelic TP53 loss due to a small mutation and cnLOH. Additionally, 9 focal CNVs (<5Mbp) were detected in 6 samples (2%). Partial tandem duplication eventsinKMT2A(MLL PTD) in 4 AML and 2 MDS samples were uniquely reported by Isabl GxT Heme. 9 SVs were reported affecting guideline genes (Table 1) .
Cytogenetics and panel sequencing information was available for 94 samples in this cohort, enabling an assessment of the validity of Isabl GxT Heme. Additional myeloid-relevant driver events not detected by panel sequencing/cytogenetics were found in 27% of patients by Isabl GxT Heme and 12% by the reference test. Isabl GxT Heme called events in 30% of patients as compared to the reference test (Fig 1). All events missed by Isabl GxT Heme were small mutations detected by panel sequencing of which 69% were absent in the aligned BAM file (11/16) and 31% (5/16) had a median variant allele fraction of 5%.
Conclusion:Isabl GxT Heme represents a myeloid cancer profiling solution that reports all clinically relevant biomarkers in AML and MDS inclusive of TP53 allelic state, MLL PTD, disease defining and risk stratifying biomarkers across mutation types. Isabl GxT Heme had comparable sensitivity to the reference test and additionally detected myeloid-relevant driver events in 27% of patients. This work demonstrates the validity and added benefit of Isabl GxT Heme in characterizing myeloid cancers.
Disclosures
Deshpande:Isabl Inc.: Current Employment, Current holder of stock options in a privately-held company. Levine:Isabl Inc.: Current Employment, Current holder of stock options in a privately-held company, Patents & Royalties. Hadi:Isabl Inc.: Current Employment, Current holder of stock options in a privately-held company. Gundem:Isabl Inc.: Consultancy. Patel:Isabl Inc.: Current Employment, Current holder of stock options in a privately-held company. Skrzypczak:Isabl Inc.: Consultancy. Kung:DarwinHealth: Membership on an entity's Board of Directors or advisory committees; Imago BioSciences: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees; Isabl Inc.: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Emendo Biotherapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Medina-Martínez:Isabl Inc.: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Papaemmanuil:TenSixteen Bio: Current equity holder in private company; Isabl Inc.: Current equity holder in private company, Current holder of stock options in a privately-held company, Other: CEO, Patents & Royalties.